Abstract
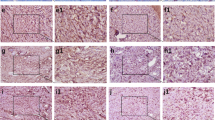
IGF-1, IGF-2, and type 1 IGF receptor (IGF-R1) mRNA expression and immunolocalization and cell proliferation index were studied in human adrenals from early infancy to late puberty. Adrenals were obtained from transplantation donors or from necropsies of endocrinologically normal subjects. Subjects were divided into three age groups: group 1, <3 mo of age, involution of fetal adrenals; group 2, 3 mo to 6 y of age, preadrenarche; and group 3, older than 6 y up to 20 y of age, postadrenarche. Cell proliferation index (Ki-67) in the outer, subcapsular, zona glomerulosa was significantly higher than in zona fasciculata of all groups and in zona reticularis or fetal zone. IGF-1 mRNA (semiquantitative reverse transcriptase–PCR and Northern blot) in group 2 was significantly higher than in group 1 and group 3 (p < 0.05). IGF2 mRNA in group 1 was significantly higher than in the other groups. IGF-R1 mRNA in group 3 was significantly higher than in group 2 but not different from group 1. Strong IGF-1, IGF-2, and IGF-R1 immunostaining signal was observed in the outer, subcapsular, zona glomerulosa and in zona fasciculata in the three groups, whereas a very weak IGF-1 and IGF-R1 immunostaining signal was found in fetal zone cells of group 1 and in zona reticularis of group 3. We propose that IGF-1 could be a factor involved in the postnatal mechanism of progenitor adrenal cell proliferation and migration. Our data also suggest that IGF-1 is not a direct regulatory factor of adrenal androgen production by zona reticularis cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CPI:
-
cell proliferation index
- DHEA:
-
dehydroepiandrosterone
- DHEAS:
-
dehydroepiandrosterone sulfate
- FeZ:
-
fetal zone IGF-R1, type 1 IGF receptor
- RT-PCR:
-
reverse transcriptase–PCR
- ZF:
-
zona fasciculata
- ZG:
-
zona glomerulosa
- ZR:
-
zona reticularis
References
Parker LN 1991 Adrenarche. Endocrinol Metab Clin North Am 20: 71–83
Zhang L, Rodriguez H, Ohno S, Miller WL 1995 Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and for the polycystic ovary syndrome. Proc Natl Acad Sci USA 92: 10619–10623
Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE 1998 Adrenarche results from development of a 3β-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab 83: 3695–3701
Dardis A, Saraco N, Rivarola MA, Belgorosky A 1999 Decrease in the expression of the 3β-hydroxysteroid dehydrogenase gene in human adrenal tissue during prepuberty and early puberty: implications to the mechanism of adrenarche. Pediatr Res 45: 384–388
Mesiano S, Jaffe RB 1997 Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18: 378–403
McNutt NS, Jones AL 1970 Observations on the ultrastructure of cytodifferentiation in the human fetal adrenal cortex. Lab Invest 22: 513–527
Wolkersdorfer G, Bornstein SR 1998 Tissue remodeling in the adrenal gland. Biochem Pharmacol 56: 163–171
Mesiano S, Coulter CL, Jaffe RB 1993 Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase/17,20-lyase, and 3β-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J Clin Endocrinol Metab 77: 1184–1189
Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ 1996 Variegated expression of a mouse steroid 21-hydroxylase/β-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol 10: 585–598
Chamoux E, Narcy A, Lehoux JG, Gallo-Payet N 2002 Fibronectin, laminin, and collagen IV as modulators of cell behavior during adrenal gland development in the human fetus. J Clin Endocrinol Metab 87: 1819–1828
Han VK, D'Ercole AJ, Lund PK 1987 Cellular localization of somatomedin (insulin-like growth factor) messenger RNA in the human fetus. Science 236: 193–197
Mesiano S, Mellow SH, Jaffe RB 1993 Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J Clin Endocrinol Metab 76: 968–976
Coulter CL, Goldsmith PC, Mesiano S, Voytek CC, Martin MC, Han VK, Jaffe RB 1996 Functional maturation of the primate fetal adrenal in vivo: I. Role of insulin-like growth factors (IGFs), IGF-1 receptor, and IGF binding proteins in growth regulation. Endocrinology 137: 4487–4498
Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A 2003 Relationship between the growth hormone/insulin-like growth factor-I axis, insulin sensitivity, and adrenal androgens in normal prepubertal and pubertal girls. J Clin Endocrinol Metab 88: 1389–1393
Guercio G, Rivarola MA, Chaler E, Maceiras M, Belgorosky A 2002 Relationship between the GH/IGF-1 axis, insulin sensitivity and adrenal androgens in normal prepubertal and pubertal boys. J Clin Endocrinol Metab 87: 1162–1169
Sucheston ME, Cannon MS 1968 Development of zonular patterns in the human adrenal gland. J Morphol 126: 477–491
Sandberg-Nordqvist AC, Stahlbom PA, Reinecke M, Collins VP, von Holst H, Sara V 1993 Characterization of insulin-like growth factor 1 in human primary brain tumors. Cancer Res 53: 2475–2478
Fichera E, Liang S, Xu Z, Guo N, Mineo R, Fujita-Yamaguchi Y 2000 A quantitative reverse transcription and polymerase chain reaction assay for human IGF-II allows direct comparison of IGF-II mRNA levels in cancerous breast, bladder, and prostate tissues. Growth Horm IGF Res 10: 61–70
Elmlinger MW, Sanatani MS, Bell M, Dannecker GE, Ranke MB 1998 Elevated insulin-like growth factor (IGF) binding protein (IGFBP)-2 and IGFBP-4 expression of leukemic T-cells is affected by autocrine/paracrine IGF-II action but not by IGF type I receptor expression. Eur J Endocrinol 138: 337–343
Peng C, Huang TH, Jeung EB, Donaldson CJ, Vale WW, Leung PC 1993 Expression of the type II activin receptor gene in the human placenta. Endocrinology 133: 3046–3049
Sambrook J, Fritsch EF, Maniatis T 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 46–52
Mc Nicol AM 1992 The human adrenal gland: aspects of structure, function and pathology. In: James VH (ed) The Adrenal Gland. Raven Press, New York, pp 1–42
Lanman JT 1953 Fetal zone of the adrenal gland; its developmental course, comparative anatomy, and possible physiological functions. Medicine (Baltimore) 32: 389–430
Baue AE, Gunther B, Hartl W, Ackenheil M, Heberer G 1984 Altered hormonal activity in severally ill patients after injury or sepsis. Arch Surg 119: 1125–1132
Sumita S 1994 Hormonal changes and pituitary histological and immunohistochemical studies of patients with multiple organ failure. Masui 10: 1541–1551
Dimopoulou I, Tsagarakis S, Anthi A, Milou E, Ilias I, Stavrakaki K, Charalambidis C, Tzanela M, Orfanos S, Mandragos K, Thalassinos N, Roussos C 2003 High prevalence of decreased cortisol reserve in brain-dead potential organ donors. Crit Care Med 31: 1113–1117
Gravame V, Porretti L, Cardillo M, Marchesi G, Rizzi M, Sacchi C, Candiani A, Chiaranda M, Gualandris L, Taioli E, Scalamogna M 1999 Hormone evaluation in brain death. Minerva Anestesiol 65: 725–731
Wang W, Yang L, Suwa T, Casson PR, Hornsby PJ 2001 Differentially expressed genes in zona reticularis cells of the human adrenal cortex. Mol Cell Endocrinol 173: 127–134
Spencer SJ, Mesiano S, Lee JY, Jaffe RB 1999 Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodeling. J Clin Endocrinol Metab 84: 1110–1115
Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y 2003 The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim Biophys Acta 1619: 317–324
Ratcliffe J, Nakanishi M, Jaffe RB 2003 Identification of definitive and fetal zone markers in the human fetal adrenal gland reveals putative developmental genes. J Clin Endocrinol Metab 88: 3272–3277
Kadowaki T, Koyasu S, Nishida E, Sakai H, Takaku F, Yahara I, Kasuga M 1986 Insulin-like growth factors, insulin, and epidermal growth factor cause rapid cytoskeletal reorganization in KB cells. Clarification of the roles of type I insulin-like growth factor receptors and insulin receptors. J Biol Chem 261: 16141–16147
Leventhal PS, Shelden EA, Kim B, Feldman EL 1997 Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J Biol Chem 272: 5214–5218
Fottner C, Engelhardt D, Weber MM 1998 Regulation of steroidogenesis by insulin-like growth factors (IGFs) in adult human adrenocortical cells: IGF-I and, more potently, IGF-II preferentially enhance androgen biosynthesis through interaction with the IGF-I receptor and IGF-binding proteins. J Endocrinol 158: 409–417
Wolf E, Kramer R, Blum WF, Foll J, Brem G 1994 Consequences of postnatally elevated insulin-like growth factor–II in transgenic mice: endocrine changes and effects on body and organ growth. Endocrinology 135: 1877–1886
Kristiansen SB, Endoh A, Casson PR, Buster JE, Hornsby PJ 1997 Induction of steroidogenic enzyme genes by insulin and IGF-I in cultured adult human adrenocortical cells. Steroids 62: 258–265
L'Allemand D, Penhoat A, Lebrethon MC, Ardèvol R, Baehr V, Oelkers W, Saez JM 1996 Insulin-like growth factors enhance steroidogenic enzyme and corticotropin receptor messenger ribonucleic acid levels and corticotropin steroidogenic responsiveness in cultured human adrenocortical cells. J Clin Endocrinol Metab 81: 3892–3897
Acknowledgements
The technical assistance of Sandra Camarero and the statistical support of Carlos Rocco and María Inés Sarchi are acknowledged. We also thank Dr. Javier Goñi for supplying organ donors adrenal tissues.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas and Fondo para la Investigación Científica y Tecnológica of Argentina, as well as by Pfizer Endocrine Care International Fund for Research and Education.
Rights and permissions
About this article
Cite this article
Baquedano, M., Berensztein, E., Saraco, N. et al. Expression of the IGF System in Human Adrenal Tissues from Early Infancy to Late Puberty: Implications for the Development of Adrenarche. Pediatr Res 58, 451–458 (2005). https://doi.org/10.1203/01.PDR.0000179392.59060.93
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000179392.59060.93
This article is cited by
-
Expression of the IGF and the aromatase/estrogen receptor systems in human adrenal tissues from early infancy to late puberty: Implications for the development of adrenarche
Reviews in Endocrine and Metabolic Disorders (2009)