Abstract
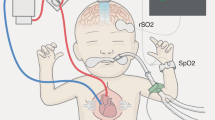
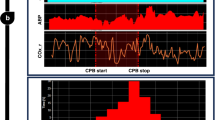
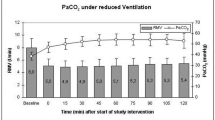
Cerebrovascular pressure autoregulation (CPA) regulates cerebral blood flow (CBF) in relation to changes in mean arterial blood pressure (MAP). Identification of a pressure-passive cerebral perfusion and the potentially modifiable physiologic factors underlying it has been difficult to achieve in sick infants. We previously validated the near-infrared spectroscopy–derived hemoglobin difference (HbD) signal (cerebral oxyhemoglobin − deoxyhemoglobin) as a reliable measure of changes in CBF in animal models. We now sought to determine whether continuous measurements of ΔHbD would correlate to middle cerebral artery flow velocity (CBFV), allow identification and quantification of pressure-passive state, and help to delineate potentially modifiable factors. We enrolled 43 infants (2 d to 7 mo old) who were undergoing open cardiac surgery and cardiopulmonary bypass. At 6 and 20 h after surgery, we measured changes in HbD, CBFV (by transcranial Doppler), and MAP at different end-tidal CO2 levels. We assigned a pressure-passive index (PPI) to each study on the basis of the relative duration of significant coherence between ΔMAP and ΔHbD. We found a significant relationship between ΔHbD and ΔCBFV at both time points. At 6 h after surgery, we showed high concordance (coherence >0.5; PPI ≥41%) between ΔMAP and ΔHbD, consistent with disturbed CPA in 13% of infants. End-tidal CO2 values ≥40 mm Hg and higher MAP variability both were associated with increased odds (p < 0.001) of autoregulatory failure. This approach provides a means to identify and quantify disturbances of CPA. High CO2 levels and fluctuating MAP are two important preventable factors associated with disturbed CPA.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CBF:
-
cerebral blood flow
- CBFV:
-
cerebral blood flow velocity
- CI:
-
confidence interval
- CPA:
-
cerebrovascular pressure autoregulation
- CV:
-
coefficient of variation
- ET-CO2:
-
end-tidal CO2
- HbD:
-
hemoglobin difference (oxyhemoglobin − deoxyhemoglobin)
- HbT:
-
hemoglobin total (oxyhemoglobin + deoxyhemoglobin)
- ICU:
-
intensive care unit
- MAP:
-
mean arterial blood pressure
- NIRS:
-
near-infrared spectroscopy
- OR:
-
odds ratio
- PPI:
-
pressure-passive index (% time pressure passive state)
- RI:
-
resistive index
- Sao2:
-
arterial oxygen saturation
- TCD:
-
transcranial Doppler
References
Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, Jonas RA, Newburger JW 1999 Developmental and neurologic status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation 100: 526–532
Majnemer A, Limperopoulos C 1999 Developmental progress of children with congenital heart defects requiring open heart surgery. Semin Pediatr Neurol 6: 12–19
du Plessis AJ, Newburger JW, Jonas RA, Wessel DL, Wypij D, Tsuji MK, Walter G, Volpe JJ 1995 Cerebral CO2 vasoreactivity is impaired in the early postoperative period following hypothermic infant cardiac surgery. [abstract]. Eur J Neurol 2: 68A
Lassen NA 1959 Cerebral blood flow and oxygen consumption in man. Physiol Rev 39: 183–233
Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ 1998 Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res 44: 591–595
Soul JS, Taylor GA, Wypij D, DuPlessis AJ, Volpe JJ 2000 Noninvasive detection of changes in cerebral blood flow by near-infrared spectroscopy in a piglet model of hydrocephalus. Pediatr Res 48: 445–449
Tsuji M, Saul JP, du Plessis AJ, Eichenwald E, Sobh J, Crocker R, Volpe JJ 2000 Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106: 625–632
du Plessis AJ, Jonas RA, Wypij D, Hickey PR, Riviello J, Wessel DL, Roth SJ, Burrows FA, Walter G, Farrell DM, Walsh AZ, Plumb CA, del Nido P, Burke RP, Castaneda AR, Mayer JE Jr Newburger JW 1997 Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg 114: 991–1001
du Plessis AJ 1995 Near-infrared spectroscopy for the in vivo study of cerebral hemodynamics and oxygenation. Curr Opin Pediatr 7: 632–639
Soul JS, du Plessis AJ 1999 New technologies in pediatric neurology. Near-infrared spectroscopy. Semin Pediatr Neurol 6: 101–110
Reinhard M, Muller T, Guschlbauer B, Timmer J, Hetzell A 2003 Transfer function analysis for clinical evaluation of dynamic cerebral autoregulation—a comparison between spontaneous and respiratory-induced oscillations. Physiol Meas 24: 27–43
Zhang R, Zuckerman JH, Giller CA, Levine BD 1998 Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol 274: H233–H241
Saul JP, Rea RF, Eckberg DL, Berger RD, Cohen RJ 1990 Heart rate and muscle sympathetic nerve variability during reflex changes of autonomic activity. Am J Physiol 258: H713–H721
Gemelli M, Manganaro R, Marni C, De Luca F 1990 Longitudinal study of blood pressure during the 1st year of life. Eur J Pediatr 149: 318–320
Pryds O, Greisen G, Skov LL, Friis-Hansen B 1990 Carbon dioxide-related changes in cerebral blood volume and cerebral blood flow in mechanically ventilated preterm neonates: comparison of near infrared spectrophotometry and 133 Xenon clearance. Pediatr Res 27: 445–449
Wyatt JS, Edwards AD, Cope M, Delpy DT, McCormick DC, Potter A, Reynolds EO 1991 Response of cerebral blood volume to changes in arterial carbon dioxide tension in preterm and term infants. Pediatr Res 29: 553–557
Kontos H 1989 Validity of cerebral blood flow calculations from velocity measurements. Stroke 20: 1–3
Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR 1994 Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke 25: 793–797
van der Linden J, Wesslen O, Ekroth R, Tyden H, von Ahn H 1991 Transcranial Doppler-estimated versus thermodilution-estimated cerebral blood flow during cardiac operations. Influence of temperature and arterial carbon dioxide tension. J Thorac Cardiovasc Surg 102: 95–102
Gilbert RD, Pearce WJ, Ashwal S, Longo LD 1990 Effects of hypoxia on contractility of isolated fetal lamb cerebral arteries. J Dev Physiol 13: 199–203
Longo LD, Pearce WJ 1991 Fetal and newborn cerebral vascular responses and adaptations to hypoxia. Semin Perinatol 15: 49–57
Smielewski P, Kirkpatrick P, Minhas P, Pickard JD, Czosnyka M 1995 Can cerebrovascular reactivity be measured with near-infrared spectroscopy?. Stroke 26: 2285–2292
Smielewski P, Czosnyka M, Pickard JD, Kirkpatrick P 1997 Clinical evaluation of near infrared spectroscopy for testing cerebrovascular reactivity in patients with carotid artery disease. Stroke 28: 331–338
Jorch G, Jorch N 1987 Failure of autoregulation of cerebral blood flow in neonates studied by pulsed Doppler ultrasound of the internal carotid artery. Eur J Pediatr 146: 468–472
Cowan F, Eriksen M, Wertheim DPF 1989 Regulation of cerebral blood flow in the newborn preterm human infant. [abstract]. J Physiol 417: 13P
Pryds O, Greisen G, Lou H, Friis-Hansen B 1990 Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr 117: 119–125
Bouma GJ, Muizelaar JP, Bandoh K, Marmarou A 1992 Blood pressure and intracranial pressure-volume dynamics in severe head injury: relationship with cerebral blood flow. J Neurosurg 77: 15–19
Tiecks FP, Lam AM, Aaslid R, Newell DW 1995 Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26: 1014–1019
Vavilala MS, Lee LA, Lam AM 2002 Cerebral blood flow and vascular physiology. Anesthesiol Clin North Am 20: 247–264
Giller CA 1990 The frequency-dependent behavior of cerebral autoregulation. Neurosurgery 27: 362–368
Panerai RB, Kelsall AW, Rennie JM, Evans DH 1996 Analysis of cerebral blood flow autoregulation in neonates. IEEE Trans Biomed Eng 43: 779–788
Panerai RB, Rennie JM, Kelsall AW, Evans DH 1998 Frequency-domain analysis of cerebral autoregulation from spontaneous fluctuations in arterial blood pressure. Med Biol Eng Comput 36: 315–322
Portnoy HD, Chopp M, Branch C, Shannon MB 1982 Cerebrospinal fluid pulse waveform as an indicator of cerebral autoregulation. J Neurosurg 56: 666–678
Menache CC, du Plessis AJ, Wessel DL, Jonas RA, Newburger JW 2002 Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg 73: 1752–1758
Haggendal E, Johansson B 1965 Effects of arterial carbon dioxide tension and oxygen saturation on cerebral blood flow autoregulation in dogs. Acta Physiol Scand Suppl 258: 27–53
Harper AM 1966 Autoregulation of cerebral blood flow: influence of the arterial blood pressure on the blood flow through the cerebral cortex. J Neurol Neurosurg Psychiatry 29: 398–403
Paulson OB, Olesen J, Christensen MS 1972 Restoration of autoregulation of cerebral blood flow by hypocapnia. Neurology 22: 286–293
Aaslid R, Lindegaard KF, Sorteberg W, Nornes H 1989 Cerebral autoregulation dynamics in humans. Stroke 20: 45–52
Newell DW, Weber JP, Watson R, Aaslid R, Winn HR 1996 Effect of transient moderate hyperventilation on dynamic cerebral autoregulation after severe head injury. Neurosurgery 39: 35–43 discussion 43–44
Smielewski P, Czosnyka M, Kirkpatrick P, McEroy H, Rutkowska H, Pickard JD 1996 Assessment of cerebral autoregulation using carotid artery compression. Stroke 27: 2197–2203
Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhaupl K, Villringer A 2000 Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 12: 623–639
Perlman JM, McMenamin JB, Volpe JJ 1983 Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med 309: 204–209
Perlman JM, Goodman S, Kreusser KL, Volpe JJ 1985 Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med 312: 1353–1357
Fujii K, Heistad DD, Faraci FM 1990 Ionic mechanisms in spontaneous vasomotion of the rat basilar artery in vivo. J Physiol (Lond) 430: 389–398
Diehl RR, Linden D, Lucke D, Berlit P 1998 Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res 8: 7–12
von Siebenthal K, Beran J, Wolf M, Keel M, Dietz V, Kundu S, Bucher HU 1999 Cyclical fluctuations in blood pressure, heart rate and cerebral blood volume in preterm infants. Brain Dev 21: 529–534
Acknowledgements
We gratefully acknowledge Dr. Joseph J. Volpe for critical review of our manuscript. We also thank Jody Bartlett and Ellen McGrath for research coordination and Shaye Moore and Michelle Mercadante for help in manuscript preparation. Many thanks to the children and their families for participating in this study.
Author information
Authors and Affiliations
Additional information
This study was supported by a grant from the LifeBridge Fund and grant HL 063411 from the National Heart, Lung, and Blood Institute.
Rights and permissions
About this article
Cite this article
Bassan, H., Gauvreau, K., Newburger, J. et al. Identification of Pressure Passive Cerebral Perfusion and Its Mediators after Infant Cardiac Surgery. Pediatr Res 57, 35–41 (2005). https://doi.org/10.1203/01.PDR.0000147576.84092.F9
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000147576.84092.F9
This article is cited by
-
Clinical applications of transcranial Doppler in non-trauma critically ill children: a scoping review
Child's Nervous System (2021)
-
Exploratory Assessment of the Relationship Between Hemoglobin Volume Phase Index, Magnetic Resonance Imaging, and Functional Outcome in Neonates with Hypoxic–Ischemic Encephalopathy
Neurocritical Care (2021)
-
Antenatal and Perioperative Mechanisms of Global Neurological Injury in Congenital Heart Disease
Pediatric Cardiology (2021)
-
Cerebral venous volume changes and pressure autoregulation in critically ill infants
Journal of Perinatology (2020)
-
Neonatal cerebrovascular autoregulation
Pediatric Research (2018)