Abstract
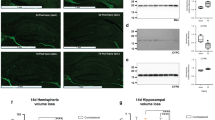
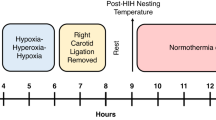
Hypoxic ischemic (HI) injury in neonates may have devastating, long-term consequences. Recently completed clinical trials in HI neonates indicate that hypothermia within 6 h of birth results in modest improvement in the combined outcome of death or severe disability. The aim of this study was to investigate the effects of combining hypothermia and N-acetylcysteine (NAC) on brain injury, neonatal reflexes and myelination after neonatal HI. Seven-day-old rats were subjected to right common carotid artery ligation and hypoxia (8% oxygen) for 2 h. Systemic hypothermia (30 + 0.5°C) was induced immediately after the period of HI and was maintained for 2 h. NAC (50 mg/kg) was administered by intraperitoneal injection daily until sacrifice. Brain infarct volumes were significantly reduced at 48 h post-HI in the hypothermia plus NAC group (21.5 ± 3.84 mm3) compared with vehicle (240.85 ± 4.08 mm3). Neonatal reflexes were also significantly improved by combination therapy at days 1 and 7. There was a significant loss of right hemispheric brain volume in the untreated group at 2 and 4 wk after HI insult. Brain volumes were preserved in hypothermia plus NAC group and were not significantly different when compared with the sham group. Similarly, increased myelin expression was seen in brain sections from hypothermia plus NAC group, when stained for Luxol Fast Blue (LFB), Myelin Basic Protein (MBP) and Proteolipid protein (PLP). These results indicate that hypothermia plus NAC combination therapy improves infarct volume, myelin expression and functional outcomes after focal HI injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- HI:
-
hypoxia-ischemia
- LFB:
-
luxol fast blue
- MBP:
-
myelin basic protein
- NAC:
-
N-acetylcysteine
- PLP:
-
proteolipid protein
References
Levene MI, Sands C, Grindulis H, Moore JR 1986 Comparison of two methods of predicting outcome in perinatal asphyxia. Lancet 1: 67–69
Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R 1991 Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev 25: 135–148
Simon NP 1999 Long-term neurodevelopmental outcome of asphyxiated newborns. Clin Perinatol 26: 767–778
Yager JY, Asselin J 1996 Effect of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke 27: 919–925
Gunn AJ, Gunn TR, De Haan HH, Williams CE, Gluckman PD 1997 Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 99: 248–256
Colbourne F, Corbett D 1994 Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res 654: 265–272
Laptook AR, Corbett RJ, Sterett R, Burns DK, Garcia D, Tollefsbol G 1997 Modest hypothermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatr Res 42: 17–23
Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY 2005 Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol 32: 11–17
Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY 2005 Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol 32: 18–24
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ 2005 Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365: 663–670
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, National Institute of Child Health and Human Development Neonatal Research Network 2005 Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353: 1574–1584
Taylor DL, Mehmet H, Cady EB, Edwards AD 2002 Improved neuroprotection with hypothermia delayed by 6 hours following cerebral hypoxia-ischemia in the 14-day-old rat. Pediatr Res 51: 13–19
Colbourne F, Sutherland GR, Auer RN 1999 Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J Neurosci 19: 4200–4210
Fairchild KD, Singh IS, Patel S, Drysdale BE, Viscardi RM, Hester L, Lazusky HM, Hasday JD 2004 Hypothermia prolongs activation of NF-{kappa}B and augments generation of inflammatory cytokines. Am J Physiol Cell Physiol 287: C422–C431
Adachi M, Sohma O, Tsuneishi S, Takada S, Nakamura H 2001 Combination effect of systemic hypothermia and caspase inhibitor administration against hypoxic-ischemic brain damage in neonatal rats. Pediatr Res 50: 590–595
Alkan T, Kahveci N, Buyukuysal L, Korfali E, Ozluk K 2001 Neuroprotective effects of MK 801 and hypothermia used alone and in combination in hypoxic-ischemic brain injury in neonatal rats. Arch Physiol Biochem 109: 135–144
Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, Singh I, Singh AK 2004 Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res 76: 519–527
Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK 2003 N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res 971: 1–8
Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ 1999 Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res 55: 158–163
Rice JE 3rd, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141
Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK 2004 N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res 78: 347–361
Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, Lengvari I 2005 Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res 157: 157–165
Reglodi D, Kiss P, Tamas A, Lengvari I 2003 The effects of PACAP and PACAP antagonist on the neurobehavioral development of newborn rats. Behav Brain Res 140: 131–139
Pabello NG, Tracy SJ, Keller RW 2004 Protective effects of brief intra- and delayed postischemic hypothermia in a transient focal ischemia model in the neonatal rat. Brain Res 995: 29–38
Zhu C, Wang X, Cheng X, Qiu L, Xu F, Simbruner G, Blomgren K 2004 Post-ischemic hypothermia-induced tissue protection and diminished apoptosis after neonatal cerebral hypoxia-ischemia. Brain Res 996: 67–75
Hermans RH, Hunter DE, Mcgivern RF, Cain CD, Longo LD 1992 Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol Teratol 14: 119–129
Tanaka S, Mito T, Takashima S 1995 Progress of myelination in the human fetal spinal nerve roots, spinal cord and brainstem with myelin basic protein immunohistochemistry. Early Hum Dev 41: 49–59
Rademaker KJ, Lam JN, Van Haastert IC, Uiterwaal CS, Lieftink AF, Groenendaal F, Grobbee DE, de Vries LS 2004 Larger corpus callosum size with better motor performance in prematurely born children. Semin Perinatol 28: 279–287
Trescher WH, Ishiwa S, Johnston MV 1997 Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev 19: 326–338
Li Y, Chopp M, Jiang N, Yao F, Zaloga C 1995 Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 15: 389–397
Grow JL, Liu YQ, Barks JD 2003 Can lateralizing sensorimotor deficits be identified after neonatal cerebral hypoxia-ischemia in rats?. Dev Neurosci 25: 394–402
Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE 2003 The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 84: 1173–1183
Acknowledgements
The authors thank Ms. Joyce Bryan and Ms. Hope Terry for their help in arrangement of animals and chemicals. We also thank Ms. Carrie Barnes for providing histopathological support. We thank the Children's Research Institute for providing the laboratory space and the animal facility for carrying out the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants (NS-40144, NS-22576, NS-34741, NS-37766, and NS-40810) from the National Institutes of Health (NIH).
Rights and permissions
About this article
Cite this article
Jatana, M., Singh, I., Singh, A. et al. Combination of Systemic Hypothermia and N-acetylcysteine Attenuates Hypoxic-Ischemic Brain Injury in Neonatal Rats. Pediatr Res 59, 684–689 (2006). https://doi.org/10.1203/01.pdr.0000215045.91122.44
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.pdr.0000215045.91122.44
This article is cited by
-
The protective effect of N-acetylcysteine against MK-801-induced neurodegeneration in mice
Molecular Biology Reports (2023)
-
Therapeutic hypothermia for the treatment of neonatal hypoxia-ischemia: sex-dependent modulation of reactive astrogliosis
Metabolic Brain Disease (2022)
-
Free radicals and neonatal encephalopathy: mechanisms of injury, biomarkers, and antioxidant treatment perspectives
Pediatric Research (2020)
-
Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy
Nano Research (2018)
-
Chronic N-acetylcysteine treatment alleviates acute lipopolysaccharide-induced working memory deficit through upregulating caveolin-1 and synaptophysin in mice
Psychopharmacology (2018)