Abstract
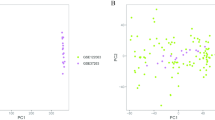
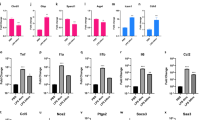
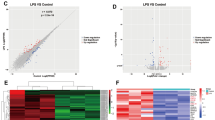
To improve the understanding of the molecular mechanisms whereby lipopolysaccharide (LPS) affects the immature brain, global gene expression following LPS exposure was investigated in neonatal rats. Brains (n = 5/time point) were sampled 2, 6, and 72 h after LPS and compared with age-matched controls. The mRNA from each brain was analyzed separately on Affymextrix GeneChip Rat Expression Set 230. The number of genes regulated after LPS were 847 at 2 h, 1564 at 6 h, and 1546 genes at 72 h. Gene ontology analysis demonstrated that, at both 2 and 6 h after LPS, genes associated with protein metabolism, response to external stimuli and stress (immune and inflammatory response, chemotaxis) and cell death were overrepresented. At 72 h, the most strongly regulated genes belonged to secretion of neurotransmitters, transport, synaptic transmission, cell migration, and neurogenesis. Several pathways associated with cell death/survival were identified (caspase-tumor necrosis factor α [TNF-α]-, p53-, and Akt/phosphatidylinositol-3-kinase (PI3 K)–dependent mechanisms). Caspase-3 activity increased and phosphorylation of Akt decreased 8 h after peripheral LPS exposure. These results show a complex cerebral response to peripheral LPS exposure. In addition to the inflammatory response, a significant number of cell death-associated genes were identified, which may contribute to increased vulnerability of the immature brain to hypoxia-ischemia (HI) following LPS exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- FAIM2:
-
fas apoptotic inhibitory molecule 2
- HI:
-
hypoxia-ischemia
- LPS:
-
lipopolysaccharide
- NF-κB:
-
nuclear factor-κB
- pAkt:
-
phosphorylated Akt
- PHLDA1:
-
pleckstrin homology-like domain, member 1
- PI3 K:
-
phosphatidylinositol-3-kinase
- PKB:
-
protein kinase B
- PND:
-
postnatal day
- RMA:
-
robust multiarray average
References
Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG 2000 Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res 47: 64–72
Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I 2003 White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res 28: 215–223
Svedin P, Kjellmer I, Welin AK, Blad S, Mallard C 2005 Maturational effects of lipopolysaccharide on white-matter injury in fetal sheep. J Child Neurol 20: 960–964
Nelson KB, Grether JK 1998 Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol 179: 507–513
Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, Hagberg H 2001 Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur J Neurosci 13: 1101–1106
Yang L, Sameshima H, Ikeda T, Ikenoue T 2004 Lipopolysaccharide administration enhances hypoxic-ischemic brain damage in newborn rats. J Obstet Gynaecol Res 30: 142–147
Ikeda T, Yang L, Ikenoue T, Mallard C, Hagberg H 2006 Endotoxin-induced hypoxic-ischemic tolerance is mediated by up-regulation of corticosterone in neonatal rat. Pediatr Res 59: 56–60
Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K 1998 CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol 8: 923–926
Hedtjarn M, Mallard C, Eklind S, Gustafson-Brywe K, Hagberg H 2004 Global gene expression in the immature brain after hypoxia-ischemia. J Cereb Blood Flow Metab 24: 1317–1332
Hedtjarn M, Mallard C, Hagberg H 2004 Inflammatory gene profiling in the developing mouse brain after hypoxia-ischemia. J Cereb Blood Flow Metab 24: 1333–1351
Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE 2003 Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci 23: 5607–5616
Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP 2003 Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15
Whitaker JR, Granum PE 1980 An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem 109: 156–159
Eklind S, Mallard C, Arvidsson P, Hagberg H 2005 Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res 58: 112–116
Nathan C 2002 Points of control in inflammation. Nature 420: 846–852
Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD, Gunn AJ 2004 TGF beta-1 and neurological function after hypoxia-ischemia in adult rats. Neuroreport 15: 961–964
Jung DY, Lee H, Jung BY, Ock J, Lee MS, Lee WH, Suk K 2005 TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-beta as a decision maker. J Immunol 174: 6467–6476
Piacentini M, Farrace MG, Piredda L, Matarrese P, Ciccosanti F, Falasca L, Rodolfo C, Giammarioli AM, Verderio E, Griffin M, Malorni W 2002 Transglutaminase overexpression sensitizes neuronal cell lines to apoptosis by increasing mitochondrial membrane potential and cellular oxidative stress. J Neurochem 81: 1061–1072
Suzuki S, Suzuki N, Mirtsos C, Horacek T, Lye E, Noh SK, Ho A, Bouchard D, Mak TW, Yeh WC 2003 Nur77 as a survival factor in tumor necrosis factor signaling. Proc Natl Acad Sci U S A 100: 8276–8280
Cui X, Imaizumi T, Yoshida H, Tanji K, Matsumiya T, Satoh K 2000 Lipopolysaccharide induces the expression of cellular inhibitor of apoptosis protein-2 in human macrophages. Biochim Biophys Acta 1524: 178–182
Myokai F, Takashiba S, Lebo R, Amar S 1999 A novel lipopolysaccharide-induced transcription factor regulating tumor necrosis factor alpha gene expression: molecular cloning, sequencing, characterization, and chromosomal assignment. Proc Natl Acad Sci U S A 96: 4518–4523
Micheau O, Tschopp J 2003 Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114: 181–190
Rensink AA, Gellekink H, Otte-Holler I, ten Donkelaar HJ, de Waal RM, Verbeek MM, Kremer B 2002 Expression of the cytokine leukemia inhibitory factor and pro-apoptotic insulin-like growth factor binding protein-3 in Alzheimer's disease. Acta Neuropathol (Berl) 104: 525–533
Devi GR, Graham DL, Oh Y, Rosenfeld RG 2001 Effect of IGFBP-3 on IGF- and IGF-analogue-induced insulin-like growth factor-I receptor (IGFIR) signalling. Growth Horm IGF Res 11: 231–239
Brywe KG, Mallard C, Gustavsson M, Hedtjarn M, Leverin AL, Wang X, Blomgren K, Isgaard J, Hagberg H 2005 IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta?. Eur J Neurosci 21: 1489–1502
Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J 2004 Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol 269: 81–94
Brustovetsky T, Antonsson B, Jemmerson R, Dubinsky JM, Brustovetsky N 2005 Activation of calcium-independent phospholipase A (iPLA) in brain mitochondria and release of apoptogenic factors by BAX and truncated BID. J Neurochem 94: 980–994
Miyazaki I, Asanuma M, Higashi Y, Sogawa CA, Tanaka K, Ogawa N 2002 Age-related changes in expression of metallothionein-III in rat brain. Neurosci Res 43: 323–333
Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J 2005 Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res 79: 522–534
Leong ML, Maiyar AC, Kim B, O'Keeffe BA, Firestone GL 2003 Expression of the serum- and glucocorticoid-inducible protein kinase, Sgk, is a cell survival response to multiple types of environmental stress stimuli in mammary epithelial cells. J Biol Chem 278: 5871–5882
Toyoshima Y, Karas M, Yakar S, Dupont J, Lee H, LeRoith D 2004 TDAG51 mediates the effects of insulin-like growth factor I (IGF-I) on cell survival. J Biol Chem 279: 25898–25904
Kim S, Domon-Dell C, Kang J, Chung DH, Freund JN, Evers BM 2004 Down-regulation of the tumor suppressor PTEN by the tumor necrosis factor-alpha/nuclear factor-kappaB (NF-kappaB)-inducing kinase/NF-kappaB pathway is linked to a default IkappaB-alpha autoregulatory loop. J Biol Chem 279: 4285–4291
Choi JS, Park HJ, Kim HY, Kim SY, Lee JE, Choi YS, Chun MH, Chung JW, Lee MY 2005 Phosphorylation of PTEN and Akt in astrocytes of the rat hippocampus following transient forebrain ischemia. Cell Tissue Res 319: 359–366
Somia NV, Schmitt MJ, Vetter DE, Van Antwerp D, Heinemann SF, Verma IM 1999 LFG: an anti-apoptotic gene that provides protection from Fas-mediated cell death. Proc Natl Acad Sci U S A 96: 12667–12672
Sole C, Dolcet X, Segura MF, Gutierrez H, Diaz-Meco MT, Gozzelino R, Sanchis D, Bayascas JR, Gallego C, Moscat J, Davies AM, Comella JX 2004 The death receptor antagonist FAIM promotes neurite outgrowth by a mechanism that depends on ERK and NF-kappa B signaling. J Cell Biol 167: 479–492
Beier CP, Wischhusen J, Gleichmann M, Gerhardt E, Pekanovic A, Krueger A, Taylor V, Suter U, Krammer PH, Endres M, Weller M, Schulz JB 2005 FasL (CD95L/APO-1L) resistance of neurons mediated by phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent expression of lifeguard/neuronal membrane protein 35. J Neurosci 25: 6765–6774
Sieburg M, Tripp A, Ma JW, Feuer G 2004 Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 tax oncoproteins modulate cell cycle progression and apoptosis. J Virol 78: 10399–10409
Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN 2005 Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene 24: 6719–6728
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Swedish Medical Research Council (K2004-33X-14185-03A and K2004-33X-09455), the Åhlén Foundation, the Sven Jerring Foundation, the Magnus Bergvall Foundation, the Wilhelm and Martina Lundgren Foundation, the Linnéa and Josef Carlsson Foundation, the Frimurare Barnhus Foundation, the Göteborg Medical Society, and the Åke Wibergs Foundation and by grants to researchers in the public health service from the Swedish government (ALF).
Rights and permissions
About this article
Cite this article
Eklind, S., Hagberg, H., Wang, X. et al. Effect of Lipopolysaccharide on Global Gene Expression in the Immature Rat Brain. Pediatr Res 60, 161–168 (2006). https://doi.org/10.1203/01.pdr.0000228323.32445.7d
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.pdr.0000228323.32445.7d
This article is cited by
-
Can Neonatal Systemic Inflammation and Hypoxia Yield a Cerebral Palsy-Like Phenotype in Periadolescent Mice?
Molecular Neurobiology (2019)
-
Role of 3-Acetyl-11-Keto-Beta-Boswellic Acid in Counteracting LPS-Induced Neuroinflammation via Modulation of miRNA-155
Molecular Neurobiology (2018)
-
Acute Neuroinflammation Promotes Cell Responses to 1800 MHz GSM Electromagnetic Fields in the Rat Cerebral Cortex
Neurotoxicity Research (2017)
-
TNFR1-JNK signaling is the shared pathway of neuroinflammation and neurovascular damage after LPS-sensitized hypoxic-ischemic injury in the immature brain
Journal of Neuroinflammation (2014)
-
Fingolimod affects gene expression profile associated with LPS-induced memory impairment
Experimental Brain Research (2014)