Abstract
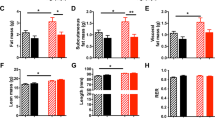
Neonatal manipulations (10 min of maternal separation plus s.c. sham injection, daily for the first 21 d of life) determine overweight in male adult mice. In this work, we investigated the mechanisms underlying mild obesity and the alteration of caloric balance. Neonatally manipulated mice become overweight after onset of maturity, showing increased fat tissue and hypertrophic epididymal adipocytes. Increase in body weight occurs in the presence of a small increase in daily food intake (significant only in the adult period) and the absence of a decrease in spontaneous locomotor activity, while the calculated caloric efficiency is higher in manipulated mice, especially in adulthood. Fasting adult animals show hyperglycemia, hyperinsulinemia, hypertriglyceridemia, hypercholesterolemia, and hyperleptinemia. Soon after weaning and in the adulthood, plasma corticosterone and adrenocorticotropin (ACTH) are also significantly increased. Thus, neonatal manipulations in nongenetically susceptible male mice program mild obesity, with metabolic and hormonal alterations that are similar to those found in experimental models of diabetes mellitus, suggesting that this metabolic derangement may have at least part of its roots early on in life and, more interestingly, that psychological and nociceptive stimuli induce these features.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ACTH:
-
adrenocorticotropin
- GC:
-
glucocorticoids
- HPA:
-
hypothalamic-pituitary-adrenal axis
- TNF-α:
-
tumor necrosis factor α
- VMH:
-
ventromedial hypothalamic nuclei
References
Godfrey KM, Barker DJ 2001 Fetal programming and adult health. Public Health Nutr 4: 611–624
Dietz WH 1994 Critical periods in childhood for the development of obesity. Am J Clin Nutr 59: 955–959
Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP 2001 The continuing epidemics of obesity and diabetes in the United States. JAMA 286: 1195–1200
Zimmet P 2003 The burden of types 2 diabetes: are we doing enough?. Diabetes Metab 29: S9–S18
Bell PM 1997 Dietary and lifestyle factors contributing to insulin resistance. Proc Nutr Soc 56: 263–272
Holness MJ, Langdown ML, Sugden MC 2000 Early-life programming of susceptibility to dysregulation of glucose metabolism and the development of type 2 diabetes mellitus. Biochem J 349: 657–665
Park IS, Bendayan M 1993 Development of the endocrine cells in the rat pancreatic and bile duct system. Histochem J 25: 807–820
Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS 2003 The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci 21: 125–132
Catargi B, Rigalleau V, Poussin A, Ronci-Chaix N, Bex V, Vergnot V, Gin H, Roger P, Tabarin A 2003 Occult Cushing's syndrome in type-2 diabetes. J Clin Endocrinol Metab 88: 5808–5813
Das UN 2002 Is type 2 diabetes mellitus a disorder of the brain?. Nutrition 18: 667–672
Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125
Kageyama A, Hirano T, Kageyama H, Osaka T, Namba Y, Tsuji M, Adachi M, Inoue S 2002 Distinct role of adiposity and insulin resistance in glucose intolerance: studies in ventromedial hypothalamic-lesioned obese rats. Metabolism 51: 716–723
Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L 2002 Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572
Carvalheira JB, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, Gontijo JA, Velloso LA, Saad MJ 2003 Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia 46: 1629–1640
D'Amore A, Mazzucchelli A, Renzi P, Loizzo A 1996 Effect of naloxone on the long- term body weight gain induced by repeated postnatal stress in male mice. Behav Pharmacol 7: 430–436
Loizzo A, Loizzo S, Lopez L, D'Amore A, Renzi P, Spampinato S, Di Carlo S, Bacosi A, Zuccaro P, Pacifici R 2002 Naloxone prevents cell-mediated immune alteration in adult mice following repeated mild stress in the neonatal period. Br J Pharmacol 135: 1219–1226
Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS 1997 Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389: 610–614
Maggisano V, Chiarotti F, Botunac I, Campanella C, Galietta G, Loizzo A 2005 Adolescence as possible critical temporal window for blood pressure short term monitoring in boys and girls. Eur J Epidemiol 20: 517–524
Heal JW 1975 An animal activity monitor using a microwave Doppler system. Med Biol Eng 13: 317
Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF 2000 Corticosterone facilitates saccharin intake in adrenalectomized rats: does corticosterone increase stimulus salience?. J Neuroendocrinol 12: 453–460
Ozanne SE 2001 Metabolic programming in animals. Br Med Bull 60: 143–152
Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M 1995 Differential effect of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 44: 645–651
Loizzo A, Capasso A, Galietta G, Severini C, Campana G, Spampinato S 2003 Vas deferens response to selective opioid receptor agonists in adult mice is impaired following postnatal repeated mild stress. Eur J Pharmacol 458: 201–205
Kalman R, Adler JH, Lazarovici G, Bar-On H, Ziv E 1993 The efficiency of sand rat metabolism is responsible for development of obesity and diabetes. J Basic Clin Physiol Pharmacol 4: 57–68
Bray GA 1995 The syndromes of obesity: an endocrine approach. In: DeGroot LJ (ed) Endocrinology. WB Saunders, Philadelphia, pp 2624–2662
Hotamisligil GS 2004 Inflammation, TNFα and insulin resistance. In: LeRoith D, Taylor SI, Olefsky JM (eds) Diabetes Mellitus: A Fundamental and Clinical Text. Lippincott Williams & Wilkins: Philadelphia
Widjaja A, Schurmeyer TH, Von zur Muhlen A, Brabant G 1998 Determinants of serum leptin levels in Cushing's syndrome. J Clin Endocrinol Metab 83: 600–603
Maffei M, Fei H, Lee GH, Dani C, Leroy P, Zhang Y, Proenca R, Negrel R, Ailhaud G, Friedman JM 1995 Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A 92: 6957–6960
Dua A, Hennes MI, Hoffmann RG, Maas DL, Krakower GR, Sonnenberg GE, Kissebah AH 1996 Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African-American women. Diabetes 45: 1635–1637
Kieffer TJ, Habener JF 2000 The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab 278: E1–E14
Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, Habener JF 1999 Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab 84: 670–676
Barden N, Reul JM, Holsboer F 1995 Do antidepressants stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system?. Trends Neurosci 18: 6–11
De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M 1997 Glucocorticoid feedback resistance. Trends Endocrinol Metab 8: 26–33
Bakker JM, van Bel F, Heijnen CJ 2001 Neonatal glucocorticoids and the developing brain: short-term treatment with life-long consequences?. Trends Neurosci 24: 649–653
Guillaume-Gentil C, Rohner-Jeanrenaud F, Abramo F, Bestetti GE, Rossi GL, Jeanrenaud B 1990 Abnormal regulation of the hypothalamo-pituitary-adrenal axis in the genetically obese fa/fa rat. Endocrinology 126: 1873–1879
Takao T, Tojo C, Nishioka T, Hashimoto K 2000 Increased adrenocorticotropin responses to acute stress in Otsuka Long-Evans Tokushima fatty (type-2 diabetic) rats. Brain Res 852: 110–115
Byrne CD 2001 Programming other hormones that affect insulin. Br Med Bull 60: 153–171
Roy MS, Roy A, Gallucci WT, Collier B, Young K, Kamilmaris TC, Chrousos GP 1993 The ovine corticotropin-releasing hormone stimulation test in type 1 diabetic patients and controls: suggestion of mild chronic hypercorticosolism. Metabolism 42: 696–700
Hudson JL, Hudson MS, Rothschild AJ, Vignati L, Schatzberg AF, Melby JC 1984 Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch Gen Psychiatry 41: 1086–1089
Strack AM, Horsley CJ, Sebastian RJ, Akana SF, Dallman MF 1995 Glucocorticoids and insulin: complex interaction on brown adipose tissue. Am J Physiol 268: R1209–R1216
Cincotta AH, Luo S, Zhang Y, Liang Y, Bina KG, Jetton TL, Scislowski PW 2000 Chronic infusion of norepinephrine into the VMH of normal rats induces the obese glucose-intolerant state. Am J Physiol Regul Integr Comp Physiol 278: R435–R444
Portha B 2003 [Transmitted beta-cell dysfunction as a cause for type 2-diabetes]. Med Sci (Paris) 19: 847–853
Lesage J, Del-Favero F, Leonhardt M, Louvart H, Maccari S, Vieau D, Darnaudery M 2004 Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol 181: 291–296
Acknowledgements
Some metabolic data were produced with the help of Dr. Antonio d'Amore and Dr. Paola Capriani. We express deep gratitude to Prof. Federico Bennardini and Dr. Maurizio Mian for advice on the manuscript, to Dr. Roberta Pacifici for leptin analysis data, to Carla Campanella for editorial work, and to Fiona Lewis for the English revision of the manuscript. Stefano Fidanza and Adriano Urcioli provided excellent animal care.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by Italian National Institute of Health Research Programs “Developmental Pharmacology” and “New Preventive and Therapeutic Approach for an Emerging Risk for the Adult Woman and Man Following Fetal and Neonatal Programming Disruption” and in part with the contribution of GIO.I.A Foundation, Pisa, and of Fondazione Cassa di Risparmio Pistoia e Pescia.
Rights and permissions
About this article
Cite this article
Loizzo, A., Loizzo, S., Galietta, G. et al. Overweight and Metabolic and Hormonal Parameter Disruption Are Induced in Adult Male Mice by Manipulations During Lactation Period. Pediatr Res 59, 111–115 (2006). https://doi.org/10.1203/01.pdr.0000190575.12965.ce
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.pdr.0000190575.12965.ce
This article is cited by
-
Early post-natal life stress induces permanent adrenocorticotropin-dependent hypercortisolism in male mice
Endocrine (2021)
-
Type 2 diabetes mellitus and psychological stress — a modifiable risk factor
Nature Reviews Endocrinology (2017)
-
The CB1 Receptor Antagonist SR141716A Reverses Adult Male Mice Overweight and Metabolic Alterations Induced by Early Stress
Obesity (2011)