Abstract
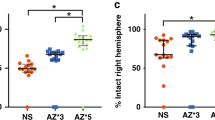
Underphysiologic conditions, brain intracellular pH (pHi) is maintained at 7.03. Rebound brain intracellular alkalosis has been observed in experimental models and adult stroke after hypoxia/ischemia (HI). In term infants with neonatal encephalopathy (NE), an association exists between the magnitude of brain alkalosis and neurodevelopmental outcome, and there is increasing evidence to suggest that alkalosis may be deleterious to cell survival. Activation of the Na+/H+ exchanger (NHE) is thought to be responsible for the rapid normalization of pHi and rebound alkalosis after reperfusion. We hypothesized that N-methyl-isobutyl-amiloride (MIA), an inhibitor of the NHE, would reduce brain injury in a model of neonatal HI. Seven-day-old mice underwent left carotid artery occlusion followed by exposure to 8% oxygen for 30 min (moderate insult) or 1 h (severe insult). Animals received MIA or saline 8 hourly starting 30 min before HI. Outcome was determined at 48 h by measuring viable tissue in the injured hemisphere (severe insult) or injury score and TUNEL staining (moderate insult). After the severe insult, MIA had a significant neuroprotective effect increasing forebrain tissue survival from 44% to 67%. After the moderate insult, damage was localized to the hippocampus where treatment resulted in a significant reduction in injury score and in TUNEL-positive cells. MIA was also shown to have a significant overall neuroprotective effect based on injury score after the moderate insult. Amiloride analogues are neuroprotective when commenced before HI in a mouse model.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- pHi:
-
intracellular pH
- MIA:
-
N-methyl-isobutyl-amiloride
- HI:
-
hypoxia/ischemia
- NHE:
-
Na+/H+ exchanger
- NE:
-
neonatal encephalopathy
References
Levene M, Evans DJ 2005 Hypoxic-ischaemic brain injury. In: Rennie JM Roberton's Textbook of Neonatology. Elsevier/Churchill Livingstone, Philadelphia 1128–1148
Hope PL, Costello AM, Cady EB, Delpy DT, Tofts PS, Chu A, Hamilton PA, Reynolds EO, Wilkie DR 1984 Cerebral energy metabolism studied with phosphorous NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 2: 366–370
Younkin DP, Delivoria-Papadopoulos M, Leonard JC, Subramanian VH, Eleff S, Leigh JS Jr, Chance B 1984 Unique aspects of human newborn cerebral metabolism evaluated with phosphorus nuclear magnetic resonance spectroscopy. Ann Neurol 16: 581–586
Laptook AR, Corbett RJ, Uauy R, Mize C, Mendelsohn D, Nunnally RL 1989 Use of 31P magnetic resonance spectroscopy to characterize evolving brain damage after perinatal asphyxia. Neurology 39: 709–712
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V, et al. 1994 Delayed (‘secondary') cerebral energy failure following acute hypoxia-ischaemia in the newborn piglet: continuous 48-hour studies by 31P magnetic resonance spectroscopy. Pediatr Res 36: 699–706
Penrice J, Lorek A, Cady EB, Amess PN, Wylezinska M, Cooper CE, D'Souza P, Brown GC, Kirkbride V, Edwards AD, Wyatt JS, Reynolds EO 1997 Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res 41: 795–802
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ 2005 Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365: 663–670
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH 2005 National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 13;353: 1574–1584
Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY 2005 Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol 32: 18–24
Masereel B, Pochet L, Laeckmann D 2003 An overview of inhibitors of Na(+)/H(+) exchanger. Eur J Med Chem 38: 547–554
Azzopardi D, Wyatt JS, Cady EB, Delpy DT, Baudin J, Stewart AL, Hope PL, Hamilton PA, Reynolds EO 1989 Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res 25: 445–451
Wakabayashi S, Fafournoux P, Sardet C, Pouyssegur J 1992 The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls “H(+)-sensing.”. Proc Natl Acad Sci U S A 89: 2424–2428
Mabe H, Blomqvist P, Siesjo BK 1983 Intracellular pH in the brain following transient ischemia. J Cereb Blood Flow Metab 31: 109–114
Chopp M, Chen H, Vande Linde AM, Brown E, Welch KM 1990 Time course of postischemic intracellular alkalosis reflects the duration of ischemia. J Cereb Blood Flow Metab 10: 860–865
Hugg JW, Duijn JH, Matson GB, Maudsley AA, Tsuruda JS, Gelinas DF, Weiner MW 1992 Elevated lactate and alkalosis in chronic human brain infarction observed by 1H and 31P MR spectroscopic imaging. J Cereb Blood Flow Metab 12: 734–744
Levine SR, Helpern JA, Welch KM, Vande Linde AM, Sawaya KL, Brown EE, Ramadan NM, Deveshwar RK, Ordidge RJ 1992 Human focal cerebral ischemia: evaluation of brain pH and energy metabolism with P-31 NMR spectroscopy. Radiology 185: 537–544
Welch KM, Levine SR, Helpern JA 1990 Pathophysiological correlates of cerebral ischemia the significance of cellular acid base shifts. Funct Neurol 5: 21–31
Robertson NJ, Cowan FM, Cox IJ, Edwards AD 2002 Brain alkaline intracellular pH after neonatal encephalopathy. Ann Neurol 52: 732–742
Robertson NJ, Cox IJ, Cowan FM, Counsell SJ, Azzopardi D, Edwards AD 1999 Cerebral intracellular lactic alkalosis persisting months after neonatal encephalopathy measured by magnetic resonance spectroscopy. Pediatr Res 46: 287–296
Ferimer HN, Kutina KL, LaManna JC 1995 Methyl isobutyl amiloride delays normalization of brain intracellular pH after cardiac arrest in rats. Crit Care Med 23: 1106–1111
Moller JC, Klein MA, Haas S, Jones LL, Kreutzberg GW, Raivich G 1996 Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia 17: 121–132
Gavrieli Y, Sherman Y, Ben-Sasson SA 1992 Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501
Ohno M, Aotani H, Shimada M 1995 Glial responses to hypoxic/ischemic encephalopathy in neonatal rat cerebrum. Brain Res Dev Brain Res 84: 294–298
Horikawa N, Nishioka M, Itoh N, Kuribayashi Y, Matsui K, Ohashi N 2001 The Na(+)/H(+) exchanger SM-20220 attenuates ischemic injury in in vitro and in vivo models. Pharmacology 63: 76–81
Vornov JJ, Thomas AG, Jo D 1996 Protective effects of extracellular acidosis and blockade of sodium/hydrogen ion exchange during recovery from metabolic inhibition in neuronal tissue culture. J Neurochem 67: 2379–2389
Robertson NJ, Bhakoo K, Puri BK, Edwards AD, Cox IJ 2005 Hypothermia and amiloride preserve energetics in a neonatal brain slice model. Pediatr Res 58: 288–296
Simon RP, Niro M, Gwinn R 1993 Brain acidosis induced by hypercarbic ventilation attenuates focal ischemic injury. J Pharmacol Exp Ther 267: 1428–1431
Vannucci RC, Towfighi J, Brucklacher RM, Vannucci SJ 2001 Effect of extreme hypercapnia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 49: 799–803
Traynelis SF, Cull-Candy SG 1990 Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 345: 347–350
Giffard RG, Weiss JH, Choi DW 1992 Extracellular alkalinity exacerbates injury of cultured cortical neurons. Stroke 23: 1817–1821
Mellgren RL 1987 Calcium-dependent proteases: an enzyme system active at cellular membranes?. FASEB J 1: 110–115
Phillis JW, Ren J, O'Regan MH 2000 Inhibition of Na(+)/H(+) exchange by 5-(N-ethyl-N-isopropyl)-amiloride reduces free fatty acid efflux from the ischemic reperfused rat cerebral cortex. Brain Res 884: 155–162
Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK 1999 Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pHi . Proc Natl Acad Sci U S A 96: 14476–14481
Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK 2001 Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport and F(O)F(1)-ATPase. J Biol Chem 276: 6453–6462
Lemasters JJ, Nieminen AL, Qian T, Trost LC, Herman B 1997 The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem 174: 159–165
Manev H, Bertolino M, DeErausquin G 1990 Amiloride blocks glutamate-operated cationic channels and protects neurons in culture from glutamate-induced death. Neuropharmacology 29: 1103–1110
Horikawa N, Kuribayashi Y, Itoh N, Nishioka M, Matsui K, Kawamura N, Ohashi N 2001 Na+/H+ exchange inhibitor SM-20220 improves endothelial dysfunction induced by ischemia-reperfusion. Jpn J Pharmacol 85: 271–277
Kintner DB, Su G, Lenart B, Ballard AJ, Meyer JW, Ng LL, Shull GE, Sun D 2004 Increased tolerance to oxygen and glucose deprivation in astrocytes from Na(+)/H(+) exchanger isoform 1 null mice. Am J Physiol Cell Physiol 287: C12–C21
Hudome S, Palmer C, Roberts RL, Mauger D, Housman C, Towfighi J 1997 The role of neutrophils in the production of hypoxic-ischemic brain injury in the neonatal rat. Pediatr Res 41: 607–616
Simchowitz L, Cragoe EJ Jr 1986 Regulation of human neutrophil chemotaxis by intracellular pH. J Biol Chem 261: 6492–6500
Fukushima T, Waddell TK, Grinstein S, Goss GG, Orlowski J, Downey GP 1996 Na+/H+ exchange activity during phagocytosis in human neutrophils: role of Fc gamma receptors and tyrosine kinases. J Cell Biol 132: 1037–1052
Simchowitz L 1985 Intracellular pH modulates the generation of superoxide radicals by human neutrophils. J Clin Invest 76: 1079–1089
Coakley RJ, Taggart C, McElvaney NG, O'Neill SJ 2002 Cytosolic pH and the inflammatory microenvironment modulate cell death in human neutrophils after phagocytosis. Blood 100: 3383–3391
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kendall, G., Robertson, N., Iwata, O. et al. N-Methyl-isobutyl-amiloride Ameliorates Brain Injury When Commenced Before Hypoxia Ischemia in Neonatal Mice. Pediatr Res 59, 227–231 (2006). https://doi.org/10.1203/01.pdr.0000196805.68082.22
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.pdr.0000196805.68082.22
This article is cited by
-
Neuroprotection of the hypoxic-ischemic mouse brain by human CD117+CD90+CD105+ amniotic fluid stem cells
Scientific Reports (2018)
-
Na+/H+ Exchangers and Intracellular pH in Perinatal Brain Injury
Translational Stroke Research (2014)
-
TNF gene cluster deletion abolishes lipopolysaccharide-mediated sensitization of the neonatal brain to hypoxic ischemic insult
Laboratory Investigation (2011)
-
Greater Hypoxia-Induced Cell Death in Prenatal Brain after Bacterial-Endotoxin Pretreatment is not Because of Enhanced Cerebral Energy Depletion: A Chicken Embryo Model of the Intrapartum Response to Hypoxia and Infection
Journal of Cerebral Blood Flow & Metabolism (2008)