Abstract
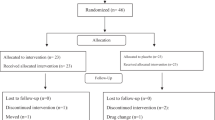
Pramlintide, a synthetic analog of amylin, improves postprandial hyperglycemia. We compared subcutaneous (s.c.) pramlintide injection with square wave pramlintide infusion in adolescents with type 1 diabetes (T1DM). Eight subjects with T1DM underwent two randomized studies. Subcutaneous pramlintide (dose = 5 μg/unit of insulin) bolus, was given one time and another time, the same dose was given as a 120-min s.c. infusion. Insulin dose was constant between studies. Gastric emptying was assessed with oral acetaminophen and [l-13C]glucose in meal. Plasma glucagon, pramlintide, and insulin concentrations were measured. Insulin concentrations (p < 0.99) between pramlintide injection versus infusion were similar; however, glucose concentrations were different (p < 0.0001), with the absence of hypoglycemia during pramlintide infusion [AUC (0–120 min) −0.07 ± 0.2 versus 1.05 ± 0.24 mg * h/dL (p < 0.0088)]. Insulin-only administration resulted in postprandial hyperglycemia and late postprandial hypoglycemia (p < 0.0001). Two subjects experienced hypoglycemia with pramlintide injection. Pramlintide bolus caused pronounced glucagon suppression (p < 0.0003) and delayed gastric emptying as ([13CO2] p < 0.0003 and acetaminophen p < 0.01) compared with infusion. We conclude that pramlintide bolus may result in an increase in risk of immediate postprandial hypoglycemia. Further modifications in pramlintide delivery are indicated before it can be safely used in children.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- HbA1c:
-
hemoglobin A1c
- T1DM:
-
type 1 diabetes
References
Gedulin BR, Rink TJ, Young AA 1997 Dose-response for glucagonostatic effect of amylin in rats. Metabolism 46: 67–70
Moore CX, Cooper GJ 1991 Co-secretion of amylin and insulin from cultured islet beta-cells: modulation by nutrient secretagogues, islet hormones and hypoglycemic agents. Biochem Biophys Res Commun 179: 1–9
Koda JE, Fineman M, Rink TJ, Dailey GE, Muchmore DB, Linarelli LG 1992 Amylin concentrations and glucose control. Lancet 339: 1179–1180
Thompson RG, Peterson J, Gottlieb A, Mullane J 1997 Effects of pramlintide, an analog of human amylin, on plasma glucose profiles in patients with IDDM: results of a multicenter trial. Diabetes 46: 632–636
Samsom M, Szarka LA, Camilleri M, Vella A, Zinsmeister AR, Rizza RA 2000 Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol Gastrointest Liver Physiol 278: G946–G951
Kong MF, King P, Macdonald IA, Blackshaw PE, Perkins AC, Armstrong E, Buchanan KD, Tattersall RB 1998 Effect of euglycaemic hyperinsulinaemia on gastric emptying and gastrointestinal hormone responses in normal subjects. Diabetologia 41: 474–481
Nyholm B, Orskov L, Hove KY, Gravholt CH, Moller N, Alberti KG, Moyses C, Kolterman O, Schmitz O 1999 The amylin analog pramlintide improves glycemic control and reduces postprandial glucagon concentrations in patients with type 1 diabetes mellitus. Metabolism 48: 935–941
Fineman M, Weyer C, Maggs DG, Strobel S, Kolterman OG 2002 The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res 34: 504–508
Heptulla RA, Rodriguez LM, Bomgaars L, Haymond MW 2005 The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes 54: 1100–1107
Kong MF, Stubbs TA, King P, Macdonald IA, Lambourne JE, Blackshaw PE, Perkins AC, Tattersall RB 1998 The effect of single doses of pramlintide on gastric emptying of two meals in men with IDDM. Diabetologia 41: 577–583
Percy AJ, Trainor DA, Rittenhouse J, Phelps J, Koda JE 1996 Development of sensitive immunoassays to detect amylin and amylin-like peptides in unextracted plasma. Clin Chem 42: 576–585
Lewanczuk RZ, Paty BW, Toth EL 2004 Comparison of the [13C]glucose breath test to the hyperinsulinemic-euglycemic clamp when determining insulin resistance. Diabetes Care 27: 441–447
Whitehouse F, Kruger DF, Fineman M, Shen L, Ruggles JA, Maggs DG, Weyer C, Kolterman OG 2002 A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 25: 724–730
Maggs DG, Fineman M, Kornstein J, Burrell T, Schwartz S, Wang Y, Ruggles JA, Kolterman OG, Weyer C 2004 Pramlintide reduces postprandial glucose excursions when added to insulin lispro in subjects with type 2 diabetes: a dose-timing study. Diabetes Metab Res Rev 20: 55–60
Rabasa-Lhoret R, Burelle Y, Ducros F, Bourque J, Lavoie C, Massicotte D, Peronnet F, Chiasson JL 2001 Use of an alpha-glucosidase inhibitor to maintain glucose homoeostasis during postprandial exercise in intensively treated Type 1 diabetic subjects. Diabet Med 18: 739–744
Fujisawa T, Ikegami H, Inoue K, Kawabata Y, Ogihara T 2005 Effect of two alpha-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 54: 387–390
Weyer C, Gottlieb A, Kim DD, Lutz K, Schwartz S, Gutierrez M, Wang Y, Ruggles JA, Kolterman OG, Maggs DG 2003 Pramlintide reduces postprandial glucose excursions when added to regular insulin or insulin lispro in subjects with type 1 diabetes: a dose-timing study. Diabetes Care 26: 3074–3079
Monsod TP, Tamborlane WV, Coraluzzi L, Bronson M, Yong-Zhan T, Ahern JA 2001 Epipen as an alternative to glucagon in the treatment of hypoglycemia in children with diabetes. Diabetes Care 24: 701–704
Haymond MW, Schreiner B 2001 Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care 24: 643–645
Stenninger E, Aman J 1993 Intranasal glucagon treatment relieves hypoglycaemia in children with type 1 (insulin-dependent) diabetes mellitus. Diabetologia 36: 931–935
Fineman MS, Koda JE, Shen LZ, Strobel SA, Maggs DG, Weyer C, Kolterman OG 2002 The human amylin analog, pramlintide, corrects postprandial hyperglucagonemia in patients with type 1 diabetes. Metabolism 51: 636–641
Kolterman OG, Schwartz S, Corder C, Levy B, Klaff L, Peterson J, Gottlieb A 1996 Effect of 14 days' subcutaneous administration of the human amylin analogue, pramlintide (AC137), on an intravenous insulin challenge and response to a standard liquid meal in patients with IDDM. Diabetologia 39: 492–499
Acknowledgements
The authors thank the GCRC nurses for the nursing assistance provided for this study. Drs. Orville Kolterman, David Maggs, and Mark Fineman were very helpful in providing the drug and pramlintide assay analysis (no monetary support was provided).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views of policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. government.
Rights and permissions
About this article
Cite this article
Rodriguez, L., Mason, K., Haymond, M. et al. The Role of Prandial Pramlintide in the Treatment of Adolescents With Type 1 Diabetes. Pediatr Res 62, 746–749 (2007). https://doi.org/10.1203/PDR.0b013e318159af8c
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e318159af8c
This article is cited by
-
A co-formulation of supramolecularly stabilized insulin and pramlintide enhances mealtime glucagon suppression in diabetic pigs
Nature Biomedical Engineering (2020)
-
Update van de farmacotherapeutische opties bij diabetes op kinderleeftijd
Nederlands Tijdschrift voor Diabetologie (2014)