Abstract
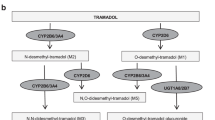
To document determinants of O-demethylation in critically ill (pre)term neonates and infants, tramadol (M) and O-demethyl tramadol (M1) concentrations were quantified in eighty-six 24 h urine collections and 168 plasma samples. A significant correlation of urine log M/M1 (0.98, SD 0.66) and plasma log M/M1 (0.78, SD 0.45) with postmenstrual age (PMA) (r = −0.69 and −0.65) was observed. One-way analysis of variance documented a significant decrease in urine log and plasma log M/M1 with increasing CYP2D6 activity score (F value 11.6 and 22.55). PMA and CYP2D6 activity score determined the urine and plasma log M/M1 (R2 0.59 and 0.64) in a forward multiple regression model. We therefore conclude that PMA and CYP2D6 polymorphisms determined O-demethylation activity in (pre)term neonates and young infants, illustrating the impact of pharmacogenetics on drug metabolism in neonates although a relevant part of the interindividual varaibility remained unexplained. Besides compound-specific relevance, CYP2D6 iso-enzyme specific data on in vivo ontogeny of O-demethylation can contribute to safer and more effective administration of drugs metabolized by the same route in this population.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- M:
-
tramadol
- M1:
-
O-demethyl tramadol
- M2:
-
N-demethyl tramadol
- PMA:
-
postmenstrual age, weeks
- PNA:
-
postnatal age, days
References
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE 2003 Developmental pharmacology: drug disposition, action, and therapy in infants and children. N Engl J Med 349: 1157–1167
Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, James LP, Wilson JT, Kearns GL, Leeder JS 2007 Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther 81: 510–516
Allegaert K, van den Anker JN, Debeer A, Cossey V, Verbesselt R, Tibboel D, Devlieger H, de Hoon J 2006 Maturational changes in the in vivo activity of CYP3A4 in the first months of life. Int J Clin Pharmacol Ther 44: 303–308
Lynn A, Nespeca MK, Bratton SL, Strauss SG, Shen DD 1998 Clearance of morphine in postoperative infants during intravenous infusion: the influence of age and surgery. Anesth Analg 86: 958–963
Carcillo JA, Doughty L, Kofos D, Frye RF, Kaplan SS, Sasser H, Burckart GJ 2003 Cytochrome P450 mediated-drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med 29: 980–984
Pedersen RS, Damkier P, Brosen K 2005 Tramadol as a new probe for cytochrome P450 2D6 phenotyping: a population study. Clin Pharmacol Ther 77: 458–467
Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F 2007 Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin Pharmacol Ther 82: 41–47
Allegaert K, van den Anker JN, Verbesselt R, de Hoon J, Vanhole C, Tibboel D, Devlieger H 2005 O-demethylation of tramadol in the first months of life. Eur J Clin Pharmacol 61: 837–842
Abdel-Rahman SM, Leeder JS, Wilson JT, Gaedigk A, Gotschall RR, Medve R, Liao S, Spielberg SP, Kearns GL 2002 Concordance between tramadol and dextromethorphan parent/metabolite ratios: the influence of CYP2D6 and non-CYP2D6 pathways on biotransformation. J Clin Pharmacol 42: 24–29
Allegaert K, Tibboel D, Naulaers G, Tison D, de Jonge A, Van Dijk M, Vanhole C, Devlieger H 2003 Systematic evaluation of pain in neonates: effect on the number of intravenous analgesics prescribed. Eur J Clin Pharmacol 59: 87–90
Allegaert K, Anderson BJ, Verbesselt R, Debeer A, de Hoon J, Devlieger H, van den Anker JN, Tibboel D 2005 Tramadol disposition in the very young: an attempt to assess in vivo cytochrome P-450 2D6 activity. Br J Anaesth 95: 231–239
Gaedigk A, Gotschall RR, Forbes NS, Simon SD, Kearns GL, Leeder JS 1999 Optimization of cytochrome P450 2D6 (CYP 2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics 9: 669–682
Hersberger M, Marti-Jaun J, Rentsch K, Hanseler E 2000 Rapid detection of the CYP2D6*3, CYP2D6*4, and CYP2D6*6 alleles by tetra-primer PCR and of the CYP2D6*5 allele by multiplex long PCR. Clin Chem 46: 1072–1077
Lovlie R, Daly AK, Molven A, Idle JR, Steen VM 1996 Ultrarapid metabolizers of debrisoquine: characterization and PCR-based detection of alleles with duplication of the CYP2D6 gene. FEBS Lett 392: 30–34
Johnson TN 2005 Different pharmacokinetics of tramadol in mothers treated for labour pain and in their neonates. Towards an increased knowledge of paediatric clinical pharmacology. Eur J Clin Pharmacol 61: 929–930
Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ 2006 Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 368: 704
Voronov P, Przybylo HJ, Jagannathan N 2007 Apnea in a child after oral codeine: a genetic variant – an ultra-rapid metabolizer. Paediatr Anaesth 17: 684–687
Brousseau DC, McGarver DG, Drendel AL, Divakaran K, Panepinto JA 2007 The effect of CYP2D6 polymorphisms on the response to pain treatment for pediatric sickle cell pain crisis. J Pediatr 150: 623–626
Özdemir M, Crewe KH, Tucker GT, Rostami-Hodjegan A 2004 Assessment of in vivo CYP2D6 activity: differential sensitivity of commonly used probes to urine pH. J Clin Pharmacol 44: 1398–1404
Johnson TN Tucker GT, Rostami-Hodjegan A Development of CYP2D6 and CYP3A4 in the first year of life. Clin Pharmacol Ther [Epub ahead of print]
Stamer UM, Lehnen K, Hothker F, Bayerer B, Wolf S, Hoeft A, Stuber F 2003 Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain 105: 231–238
Sachse C, Brockmoller J, Bauer S, Roots I 1997 Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 60: 284–295
Garrido MJ, Habre W, Rombout F, Trocóniz IF 2006 Population pharmacokinetic/pharmacodynamic modelling of the analgesic effects of tramadol in pediatrics. Pharm Res 23: 2014–2023
van den Anker JN 1997 Renal function in preterm infants. Eur J Pediatr 156: 583–584
Treszl A, Kaposi A, Hajdu J, Szabo M, Tulassay T, Vasarhelyi B 2007 The extent to which genotype information may add to the prediction of disturbed perinatal adaptation: none, minor or major?. Pediatr Res 62: 610–614
Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR Jr, Vazquez DM 2005 Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol 25: 595–604
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Clinical Research Fund of the University Hospitals Leuven, Belgium (to K.A.), and by grant HD45993 (NICHD) and RR19729 (NCRR) (to J.N.v.d.A.).
Rights and permissions
About this article
Cite this article
Allegaert, K., van Schaik, R., Vermeersch, S. et al. Postmenstrual Age and CYP2D6 Polymorphisms Determine Tramadol O-demethylation in Critically Ill Neonates and Infants. Pediatr Res 63, 674–679 (2008). https://doi.org/10.1203/PDR.0b013e31816ff712
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e31816ff712
This article is cited by
-
Precision therapeutics in the NICU: why are we missing the mark?
Journal of Perinatology (2018)
-
Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates
Pediatric Research (2017)
-
The Pharmacogenetics of Tramadol
Clinical Pharmacokinetics (2015)
-
Mechanism based medicine in infancy: complex interplay between developmental pharmacology and pharmacogenetics
International Journal of Clinical Pharmacy (2011)
-
Maternal–fetal and neonatal pharmacogenomics: a review of current literature
Journal of Perinatology (2010)