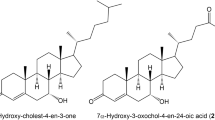
Abstract
Retention of bile acids within the liver is a primary factor in the pathogenesis of cholestatic liver disorders, which are more common in human infants. The objective of this study was to evaluate developmental changes in mitochondrial factors involved in bile acid-induced hepatocyte injury. Hepatic mitochondria from adult rats (aged 9 wk) underwent a mitochondrial permeability transition (MPT) and release of cytochrome c upon exposure to glycochenodeoxycholic acid. In contrast, mitochondria from young rats (age 6–36 d) were resistant to MPT induction and cytochrome c release. Neither mitochondrial levels of MPT-associated proteins (voltage-dependent anion channel, cyclophilin D, or adenine nucleotide translocase), Bcl-2 family proteins, nor antioxidant enzymes explained this resistance. Mitochondria from young rats contained 2- to 3-fold higher α-tocopherol (α-TH). In vivo α-TH enrichment of adult hepatic mitochondria increased their MPT resistance. Tetra-linoleoyl cardiolipin (TL-CL), the primary molecular species of CL, was reduced in mitochondria of the young rat; however, enrichment with CL and TL-CL only modestly increased their MPT susceptibility. In conclusion, we observed an unexpected resistance in young rats to bile acid induction of mitochondrial cell death pathways, which may be related to developmental differences in membrane composition.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- α-TH:
-
alpha-tocopherol
- α-TTP:
-
alpha-tocopherol transfer protein
- CL:
-
cardiolipin
- GSH-Px:
-
glutathione peroxidase
- GSSG-R:
-
glutathione reductase
- GCDC:
-
glycochenodeoxycholic acid
- MPT:
-
mitochondrial permeability transition
- ROS:
-
reactive oxygen species
- TL-CL:
-
tetra-linoleoyl CL
References
Arrese M, Ananthananarayanan M, Suchy FJ 1998 Hepatobiliary transport: molecular mechanisms of development and cholestasis. Pediatr Res 44: 141–147
Phillips MJ 1994 Mechanisms and morphology of cholestasis. Suchy FJ Liver Disease in Children. St. Louis, MO, Moseby 129–144
Pittschieler K, Lebenthal E, Bujanover Y, Petell JK 1991 Levels of Cu-Zn and Mn superoxide dismutases in rat liver during development. Gastroenterology 100: 1062–1068
Bohles H 1997 Antioxidative vitamins in prematurely and maturely born infants. Int J Vitam Nutr Res 67: 321–328
Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM 2000 Bile acids affect liver mitochondrial bioenergetics: possible relevance for cholestasis therapy. Toxicol Sci 57: 177–185
Krahenbuhl S, Talos C, Sven F, Reichen J 1994 Toxicity of bile acids on the electron transport chain in isolated rat liver mitochondria. Hepatology 19: 471–479
Krahenbuhl S, Talos C, Reichen J 1994 Mechanisms of impaired hepatic fatty acid metabolism in rats with long-term bile duct ligation. Hepatology 19: 1272–1281
Palmeira CM, Rolo AP 2004 Mitochondrially-mediated toxicity of bile acids. Toxicology 203: 1–15
Sokol RJ, Straka MS, Dahl R, Devereaux MW, Yerushalmi B, Gumpricht E, Elkins N, Everson G 2001 Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr Res 49: 519–531
Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ 1998 Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med 4: 165–178
Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ 1999 Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ 6: 842–854
Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ 2000 Bile salts mediate hepatocytes apoptosis by increasing cell surface trafficking of fas. Am J Physiol Gastrointest Liver Physiol 278: G992–G999
Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ 1999 Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 117: 669–677
Cheng X, Buckley D, Klaassen CD 2007 Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol 74: 1665–1676
Lawrence RA, Burk RF 1976 Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71: 952–958
Carlberg I, Mannervik B 1985 Glutathione reductase. Methods Enzymol 113: 484–490
Lang JK, Gohil K, Packer L 1986 Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem 157: 106–116
Podda M, Weber C, Traber MG, Packer L 1996 Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res 37: 893–901
Gumpricht E, Dahl R, Devereaux MW, Sokol RJ 2005 Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem 280: 10556–10563
Gumpricht E, Devereaux MW, Traber M, Sokol RJ 2004 Enrichment of rat hepatic organelles by vitamin E administered subcutaneously. Free Radic Biol Med 37: 1712–1717
Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC 2005 Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res 46: 1196–1204
Hackenbrock CR, Chazotte B 1986 Lipid enrichment and fusion of mitochondrial inner membranes. Methods Enzymol 125: 35–45
Belknap WM, Balistreri WF, Suchy FJ, Miller PC 1981 Physiologic cholestasis II: serum bile acid levels reflect the development of the enterohepatic circulation in rats. Hepatology 1: 613–616
Rippin SJ, Hagenbuch B, Meier PJ, Stieger B 2001 Cholestatic expression pattern of sinusoidal and canalicular organic anion transport systems in primary cultured rat hepatocytes. Hepatology 33: 776–782
Gao B, St Pierre MV, Stieger B, Meier PJ 2004 Differential expression of bile salt and organic anion transporters in developing rat liver. J Hepatol 41: 201–208
Kurosawa H, Que FG, Roberts LR, Fesmier PJ, Gores GJ 1997 Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol 272: G1587–G1593
Janiak F, Leber B, Andrews DW 1994 Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J Biol Chem 269: 9842–9849
Motoyama S, Kitamura M, Saito S, Minamiya Y, Suzuki H, Saito R, Terada K, Ogawa J, Inaba H 1998 Bcl-2 is located predominantly in the inner membrane and crista of mitochondria in rat liver. Biochem Biophys Res Commun 249: 628–636
Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ 2001 Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology 33: 616–626
Sokol RJ, Dahl R, Devereaux MW, Yerushalmi B, Kobak GE, Gumpricht E 2005 Human hepatic mitochondria generate reactive oxygen species and undergo the permeability transition in response to hydrophobic bile acids. J Pediatr Gastroenterol Nutr 41: 235–243
Kim HS, Arai H, Arita M, Sato Y, Ogihara T, Tamai H, Inoue K, Mino M 1996 Age-related changes of alpha-tocopherol transfer protein expression in rat liver. J Nutr Sci Vitaminol (Tokyo) 42: 11–18
Traber MG, Burton GW, Hamilton RL 2004 Vitamin E trafficking. Ann N Y Acad Sci 1031: 1–12
Chicco AJ, Sparagna GC 2007 Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol 292: C33–C44
Matsko CM, Hunter OC, Rabinowich H, Lotze MT, Amoscato AA 2001 Mitochondrial lipid alterations during Fas- and radiation-induced apoptosis. Biochem Biophys Res Commun 287: 1112–1120
Lieser MJ, Park J, Natori S, Jones BA, Bronk SF, Gores GJ 1998 Cholestasis confers resistance to the rat liver mitochondrial permeability transition. Gastroenterology 115: 693–701
Basova LV, Kurnikov IV, Wang L, Ritov VB, Belikova NA, Vlasova II, Pacheco AA, Winnica DE, Peterson J, Bayir H, Waldeck DH, Kagan VE 2007 Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry 46: 3423–3434
Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, DeKosky ST, Greenberger JS, Ahvedova AA, Kagan VE 2006 Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochim Biophys Acta 1757: 648–659
Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG 2005 Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1: 223–232
Petrosillo G, Casanova G, Matera M, Ruggiero FM, Paradies G 2006 Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett 580: 6311–6316
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by grants to RJS from the NIH (RO1DK038446); The Children's Hospital Research Institute and the Abby Bennet Liver Research Fund, and grants to MGT from the NIH (RO1DK59576) and the Environmental Health Sciences Center at Oregon State University (NIEHS P30 ES00210).Presented in part at the 56th Annual Meeting of the American Association for the Study of Liver Diseases, San Francisco, CA, November 2005, and published in abstract form (Hepatology 2005; 777A); and at the 58th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, MA, November 2007 and published in abstract form (Hepatology 2007: 516 A).Supplemental materials, which accompany this article, are available online at http://www.pedresearch.org
ArticlePlus
Click on the links below to access all the ArticlePlus for this article.
Please note that ArticlePlus files may launch a viewer application outside of your web browser.
Rights and permissions
About this article
Cite this article
Gumpricht, E., Devereaux, M., Dahl, R. et al. Resistance of Young Rat Hepatic Mitochondria to Bile Acid-Induced Permeability Transition: Potential Role of α-Tocopherol. Pediatr Res 64, 498–504 (2008). https://doi.org/10.1203/PDR.0b013e3181841ee1
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181841ee1