Abstract
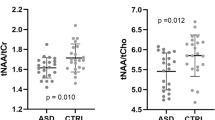
Prematurity is associated with volumetric reductions in specific brain areas such as the hippocampus and with metabolic changes that can be detected by spectroscopy. Short echo time (35 ms) Proton magnetic resonance spectroscopy (1H MRS) was performed to assess possible medial temporal lobe metabolic abnormalities in 21 adolescents with preterm birth (mean age: 14.8, SD: 1.3) compared with an age-matched control sample (mean age: 14.8, SD: 1.6). 1H MRS spectra were analyzed with linear combination model fitting, obtaining the absolute metabolite concentrations for Creatine (Cr), and myo-inositol (Ins). In addition, the following metabolite sums were measured: total Cho (glycerophospho-choline + phosphocholine), total N-acetyl-aspartate + N-acetyl-aspartylglutamate (NA), and total Glx (glutamate + glutamine). A stereological analysis was performed to calculate hippocampal volume. Absolute Cr, and total NA values were decreased in the preterm group (p = 0.016; p = 0.002, respectively). The preterm also showed a hippocampal reduction (p < 0.0001). Significant relationships were found between gestational age and different metabolites and the hippocampal volume. Moreover, hippocampal volume correlated with brain metabolites in the whole sample. Results demonstrate that prematurity affects medial temporal lobe metabolites, and that the alteration is related to structural changes, suggesting that the cerebral changes persist until adolescence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- Cho:
-
choline
- Cr:
-
creatine
- CSF:
-
cerebral spinal fluid
- Glx:
-
glutamate + glutamine
- GM:
-
gray matter
- 1H MRS:
-
proton magnetic resonance spectroscopy
- ICV:
-
intracranial volume, Ins, myo-inositol
- NA:
-
N-acetyl-aspartate + N-acetyl-aspartylglutamate
- NAA:
-
N-acetyl-aspartate
- TE:
-
echo time
- VOI:
-
volume of interest
References
Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, Ebbitt TB, Duncan CC, Makuch RW, Reiss AL 2004 Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol 31: 318–325
Nosarti C, Al-Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM 2002 Adolescents who were born very preterm have decreased brain volumes. Brain 125: 1616–1623
Gimenez M, Junque C, Narberhaus A, Caldu X, Salgado-Pineda P, Bargallo N, Segarra D, Botet F 2004 Hippocampal gray matter reduction associates with memory deficits in adolescents with history of prematurity. Neuroimage 23: 869–877
Huppi PS, Schuknecht B, Boesch C, Bossi E, Felblinger J, Fusch C, Herschkowitz N 1996 Structural and neurobehavioral delay in postnatal brain development of preterm infants. Pediatr Res 39: 895–901
Isaacs EB, Lucas A, Chong WK, Wood SJ, Johnson CL, Marshall C, Vargha-Khadem F, Gadian DG 2000 Hippocampal volume and everyday memory in children of very low birth weight. Pediatr Res 47: 713–720
Gimenez M, Junque C, Vendrell P, Caldu X, Narberhaus A, Bargallo N, Falcon C, Botet F, Mercader JM 2005 Hippocampal functional magnetic resonance imaging during a face-name learning task in adolescents with antecedents of prematurity. Neuroimage 25: 561–569
Gimenez M, Junque C, Narberhaus A, Botet F, Bargallo N, Mercader JM 2006 Correlations of thalamic reductions with verbal fluency impairment in prematures. Neuroreport 17: 463–466
Hofmann L, Slotboom J, Jung B, Maloca P, Boesch C, Kreis R 2002 Quantitative 1H magnetic resonance spectroscopy of human brain: Influence of composition and parameterization of the basis set in linear combination model-fitting. Magn Reson Med 48: 440–453
Soher BJ, Young K, Govindaraju V, Maudsley AA 1998 Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med 40: 822–831
Zhong K, Ernst T 2004 Localized in vivo human 1H MRS at very short echo times. Magn Reson Med 52: 898–901
Provencher SW 1993 Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30: 672–679
Provencher SW 2001 Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 14: 260–264
Klose U 1990 In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med 14: 26–30
Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA 2006 Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55: 1219–1226
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW 1996 Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93: 3908–3913
Urenjak J, Williams SR, Gadian DG, Noble M 1992 Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem 59: 55–61
Demougeot C, Garnier P, Mossiat C, Bertrand N, Giroud M, Beley A, Marie C 2001 N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J Neurochem 77: 408–415
Bertolino A, Weinberger DR 1999 Proton magnetic resonance spectroscopy in schizophrenia. Eur J Radiol 30: 132–141
Farchione TR, Moore GJ, Rosenberg DR 2002 Proton magnetic resonance spectroscopic imaging in pediatric major depression. Biol Psychiatry 52: 86–92
Block W, Träber F, Flacke S, Jessen F, Pohl C, Schild H 2002 In-vivo proton MR-spectroscopy of the human brain: Assessment of N-acetylaspartate (NAA) reduction as a marker for neurodegeneration. Amino Acids 23: 317–323
Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, Ferriero DM 1999 Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol 20: 1399–1405
Wyss M, Kaddurah-Daouk R 2000 Creatine and creatinine metabolism. Physiol Rev 80: 1107–1213
Holtzman D, Togliatti A, Khait I, Jensen F 1998 Creatine increases survival and suppresses seizures in the hypoxic immature rat. Pediatr Res 44: 410–414
Maier M, Ron MA, Barker GJ, Tofts PS 1995 Proton magnetic resonance spectroscopy: an in vivo method of estimating hippocampal neuronal depletion in schizophrenia. Psychol Med 25: 1201–1209
Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, Singer E, Cornford M 1997 Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology 48: 836–845
Toft PB, Leth H, Lou HC, Pryds O, Henriksen O 1994 Metabolite concentrations in the developing brain estimated with proton MR spectroscopy. J Magn Reson Imaging 4: 674–680
Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS 2002 Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 48: 949–958
Sawrie SM, Martin RC, Knowlton R, Faugth E, Gilliam F, Kuzniecky R 2001 Relationships among hippocampal volumetry, proton magnetic resonance spectroscopy, and verbal memory in temporal lobe epilepsy. Epilepsia 42: 1403–1407
Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW 1999 Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging 20: 279–285
Anand KJ 2000 Effects of perinatal pain and stress. Prog Brain Res 122: 117–129
Poland RE, Cloak C, Lutchmansingh PJ, McCracken JT, Chang L, Ernst T 1999 Brain N-acetyl aspartate concentrations measured by H MRS are reduced in adult male rats subjected to perinatal stress: preliminary observations and hypothetical implications for neurodevelopmental disorders. J Psychiatr Res 33: 41–51
Berger R, Middelanis J, Vaihinger HM, Mies G, Wilken B, Jensen A 2004 Creatine protects the immature brain from hypoxic-ischemic injury. J Soc Gynecol Investig 11: 9–15
Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR 2000 Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284: 1939–1947
Nosarti C, Allin MP, Frangou S, Rifkin L, Murray RM 2005 Hyperactivity in adolescents born very preterm is associated with decreased caudate volume. Biol Psychiatry 57: 661–666
Rademaker KJ, Rijpert M, Uiterwaal CS, Lieftink AF, van Bel F, Grobbee DE, de Vries LS, Groenendaal F 2006 Neonatal Hydrocortisone treatment related to 1H MRS of the hippocampus and short-term memory at school age in preterm born children. Pediatr Res 59: 309–313
Traber F, Block W, Freymann N, Gur O, Kucinski T, Hammen T, Ende G, Pilatus U, Hampel H, Schild HH, Heun R, Jessen F 2006 A multicenter reproducibility study of single-voxel (1)H MRS of the medial temporal lobe. Eur Radiol 16: 1096–1103
Hammen T, Stadlbauer A, Tomandl B, Ganslandt O, Pauli E, Huk W, Neundorfer B, Stefan H 2005 Short TE single-voxel 1H MR spectroscopy of hippocampal structures in healthy adults at 1.5 Tesla–how reproducible are the results?. NMR Biomed 18: 195–201
McLean MA, Woermann FG, Simister RJ, Barker GJ, Duncan JS 2001 In vivo short echo time 1H magnetic resonance spectroscopic imaging (MRSI) of the temporal lobes. Neuroimage 14: 501–509
Author information
Authors and Affiliations
Additional information
Supported by grants from the Ministerio de Ciencia y Tecnología (SAF2005-007340), and the Generalitat de Catalunya (2005 SGR 00855). M.G. and S.S.-P. hold a grant from the Ministerio de Educación, Cultura y Deporte (AP2002-0737 and AP2005-0047, respectively).
Rights and permissions
About this article
Cite this article
Gimenez, M., Soria-Pastor, S., Junque, C. et al. Proton Magnetic Resonance Spectroscopy Reveals Medial Temporal Metabolic Abnormalities in Adolescents With History of Preterm Birth. Pediatr Res 64, 572–577 (2008). https://doi.org/10.1203/PDR.0b013e3181841eab
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181841eab
This article is cited by
-
Neurometabolic changes in neonates with congenital heart defects and their relation to neurodevelopmental outcome
Pediatric Research (2023)
-
Reduced hippocampal subfield volumes and memory performance in preterm children with and without germinal matrix-intraventricular hemorrhage
Scientific Reports (2021)
-
Altered brain metabolism contributes to executive function deficits in school-aged children born very preterm
Pediatric Research (2020)
-
Aberrant structural and functional connectivity and neurodevelopmental impairment in preterm children
Journal of Neurodevelopmental Disorders (2018)
-
The long-term effect of erythropoiesis stimulating agents given to preterm infants: a proton magnetic resonance spectroscopy study on neurometabolites in early childhood
Pediatric Radiology (2018)