Abstract
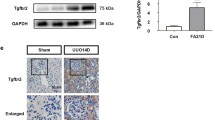
Conditional deletion of murine fibroblast growth factor receptors (Fgfrs) 1 and 2 in metanephric mesenchyme leads to renal agenesis with unbranched ureteric buds; however, there are occasionally two buds per nephric duct. Our goal was to determine whether conditional deletion of Fgfr1 or Fgfr2 alone resulted in multiple ureteric bud induction sites. Although deletion of Fgfr1 alone results in no abnormalities, loss of Fgfr2 often leads to multiple ureteric buds and anomalies including renal aplasia, misshaped kidneys, partially duplicated kidneys, duplicated ureters, and obstructed hydroureter. Deletion of Fgfr2 did not change expression domains of glial cell line-derived neurotrophic factor (GDNF), Robo2, bone morphogenetic protein 4, or Sprouty1, all of which regulate ureteric bud induction. Cultured Fgfr2 mutant nephric ducts were also not more sensitive to exogenous GDNF than controls. Whole mount in situ hybridization revealed that in mutant embryos, Fgfr2 was deleted from stromal cells around the nephric duct and ureteric bud base, which correlates well with the ureteric bud induction abnormalities. Thus, Fgfr2 is critical in ensuring that there is a single ureteric bud from the nephric duct. The plethora of later stage defects in Fgfr2 conditional knockouts is reminiscent of many human cases of genetic urogenital anomalies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- Bmp4:
-
bone morphogenetic protein 4
- E:
-
embryonic
- Fgfr :
-
fibroblast growth factor receptor
- Fgfr Mes−/− :
-
conditional deletion of Fgfr from the metanephric mesenchyme
- Fgfr UB−/− :
-
conditional deletion of Fgfr from the ureteric bud
- GDNF:
-
glial cell-line derived neurotrophic factor
References
Powers CJ, McLeskey SW, Wellstein A 2000 Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7: 165–197
Dressler GR 2006 The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529
Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D 1997 Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol 273: F757–F767
Perantoni AO, Dove LF, Karavanova I 1995 Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci USA 92: 4696–4700
Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G 1998 Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J 17: 1642–1655
Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N 2000 FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277: 643–649
Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D 1999 FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126: 547–554
Revest JM, Spencer-Dene B, Kerr K, De Moerlooze L, Rosewell I, Dickson C 2001 Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol 231: 47–62
Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR 2005 FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132: 3847–3857
Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M 2005 Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132: 3859–3871
Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P 1998 Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA 95: 5082–5087
Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P 1994 Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8: 3045–3057
Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C 1998 Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125: 753–765
Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J 1994 fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev 8: 3032–3044
Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM 2004 Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415
Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM 2006 Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291: 325–339
Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD 2005 Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8: 229–239
Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R 2007 Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signaling during kidney branching morphogenesis. Development 134: 2397–2405
Sims-Lucas S, Caruana G, Dowling J, Kett MM, Bertram JF 2008 Augmented and accelerated nephrogenesis in TGF-beta2 heterozygous mutant mice. Pediatr Res 63: 607–612
Grieshammer U, Le M, Plump AS, Wang F, Tessier-Lavigne M, Martin GR 2004 SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 6: 709–717
Kume T, Deng K, Hogan BL 2000 Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127: 1387–1395
Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I 2000 Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873
Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL 2005 Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 37: 1082–1089
Cohen MM Jr Kreiborg S 1993 Visceral anomalies in the Apert syndrome. Am J Med Genet 45: 758–760
Passos-Bueno MR, Wilcox WR, Jabs EW, Sertie AL, Alonso LG, Kitoh H 1999 Clinical spectrum of fibroblast growth factor receptor mutations. Hum Mutat 14: 115–125
Arfeen S, Rosborough D, Luger AM, Nolph KD 1993 Familial unilateral renal agenesis and focal and segmental glomerulosclerosis. Am J Kidney Dis 21: 663–668
Murugasu B, Cole BR, Hawkins EP, Blanton SH, Conley SB, Portman RJ 1991 Familial renal adysplasia. Am J Kidney Dis 18: 490–494
Schwaderer AL, Bates CM, McHugh KM, McBride KL 2007 Renal anomalies in family members of infants with bilateral renal agenesis/adysplasia. Pediatr Nephrol 22: 52–56
Roodhooft AM, Birnholz JC, Holmes LB 1984 Familial nature of congenital absence and severe dysgenesis of both kidneys. N Engl J Med 310: 1341–1345
Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR 1995 Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 9: 358–364
Carmi R, Binshtock M, Abeliovich D, Bar-Ziv J 1983 The branchio-oto-renal (BOR) syndrome: report of bilateral renal agenesis in three sibs. Am J Med Genet 14: 625–627
Acknowledgements
We thank Dr. Jon Epstein for the Pax3cre mice, Dr. Janet Rossant for the floxed Fgfr1 mice, and Dr. David Ornitz for the floxed Fgfr2 mice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NIH R01 DK070030–01 (to C.M.B.).
Rights and permissions
About this article
Cite this article
Hains, D., Sims-Lucas, S., Kish, K. et al. Role of Fibroblast Growth Factor Receptor 2 in Kidney Mesenchyme. Pediatr Res 64, 592–598 (2008). https://doi.org/10.1203/PDR.0b013e318187cc12
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e318187cc12
This article is cited by
-
Loss of peri-Wolffian duct stromal Frs2α expression in mice leads to abnormal ureteric bud induction and vesicoureteral reflux
Pediatric Research (2017)
-
Genome-wide linkage and association study implicates the 10q26 region as a major genetic contributor to primary nonsyndromic vesicoureteric reflux
Scientific Reports (2017)
-
Fibroblast growth factor receptor signaling in kidney and lower urinary tract development
Pediatric Nephrology (2016)
-
Vesicoureteric reflux and reflux nephropathy: from mouse models to childhood disease
Pediatric Nephrology (2014)