Abstract
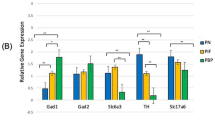
Prenatal nicotine exposure influences neuronal development including effects on several neurotransmitter systems. It also attenuates the ventilatory response to hypoxia, known to require a functional substance P-ergic system. Previous studies have shown that nicotine increases the risk for sudden infant death syndrome (SIDS) by 4-fold, and that SIDS-victims have elevated brainstem levels of substance P. We, therefore, studied the effect of prenatal nicotine exposure on the levels of substance P-like immunoreactivity by RIA in the brain in newborn rat pups. The expression of the substance P precursor preprotachykinin A mRNA was also determined by real-time reverse transcriptase-polymerase chain reaction in carotid body, in petrosal/jugular and trigeminal ganglia, in cervical and lumbar dorsal root ganglia, and in the brainstem. We found that prenatal nicotine exposure increased levels of substance P-like immunoreactivity in the brainstem without changing levels in other parts of the brain or in the adrenals. Furthermore, mRNA levels were increased in the carotid bodies and in the petrosal ganglia, in contrast to the decreased levels in the cervical dorsal root ganglia. We conclude that nicotine causes alterations in the substance P-ergic system in the brainstem, possibly linked to the increased risk for SIDS after prenatal nicotine exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DRGs:
-
dorsal root ganglia
- NK-1:
-
neurokinin 1-receptor
- NTS:
-
nucleus tractus solitarii
- RT-PCR:
-
reverse transcriptase-polymerase chain reaction
- PPT-A:
-
preprotachykinin A
References
Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, Slotkin TA 1989 Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther 251: 894–900
Wickström R 2007 Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol 5: 213–222
Holgert H, Hokfelt T, Hertzberg T, Lagercrantz H 1995 Functional and developmental studies of the peripheral arterial chemoreceptors in rat: effects of nicotine and possible relation to sudden infant death syndrome. Proc Natl Acad Sci U S A 92: 7575–7579
Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ 1995 Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for sudden infant death syndrome. Brain Res Bull 38: 69–75
Fewell JE, Smith FG 1998 Perinatal nicotine exposure impairs ability of newborn rats to autoresuscitate from apnea during hypoxia. J Appl Physiol 85: 2066–2074
Gauda EB, Cooper R, Akins PK, Wu G 2001 Prenatal nicotine affects catecholamine gene expression in newborn rat carotid body and petrosal ganglion. J Appl Physiol 91: 2157–2165
Dani JA, Heinemann S 1996 Molecular and cellular aspects of nicotine abuse. Neuron 16: 905–908
Yamamoto Y, Lagercrantz H 1985 Some effects of substance P on central respiratory control in rabbit pups. Acta Physiol Scand 124: 449–455
Monteau R, Ptak K, Broquere N, Hilaire G 1996 Tachykinins and central respiratory activity: an in vitro study on the newborn rat. Eur J Pharmacol 314: 41–50
Yamamoto Y, Onimaru H, Homma I 1992 Effect of substance P on respiratory rhythm and pre-inspiratory neurons in the ventrolateral structure of rostral medulla oblongata: an in vitro study. Brain Res 599: 272–276
Berner J, Shvarev Y, Lagercrantz H, Bilkei-Gorzo A, Hokfelt T, Wickstrom R 2007 Altered respiratory pattern and hypoxic response in transgenic newborn mice lacking the tachykinin-1 gene. J Appl Physiol 103: 552–559
Wickstrom HR, Berner J, Holgert H, Hokfelt T, Lagercrantz H 2004 Hypoxic response in newborn rat is attenuated by neurokinin-1 receptor blockade. Respir Physiol Neurobiol 140: 19–31
Feldman JL, Mitchell GS, Nattie EE 2003 Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266
Bergstrom L, Lagercrantz H, Terenius L 1984 Post-mortem analyses of neuropeptides in brains from sudden infant death victims. Brain Res 323: 279–285
Ptak K, Di Pasquale E, Monteau R 1999 Substance P and central respiratory activity: a comparative in vitro study on foetal and newborn rat. Brain Res Dev Brain Res 114: 217–227
Quirion R, Dam TV 1986 Ontogeny of substance P receptor binding sites in rat brain. J Neurosci 6: 2187–2199
Viemari JC, Burnet H, Bevengut M, Hilaire G 2003 Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur J Neurosci 17: 1233–1244
Srinivasan M, Goiny M, Pantaleo T, Lagercrantz H, Brodin E, Runold M, Yamamoto Y 1991 Enhanced in vivo release of substance P in the nucleus tractus solitarii during hypoxia in the rabbit: role of peripheral input. Brain Res 546: 211–216
Wickstrom HR, Holgert H, Hokfelt T, Lagercrantz H 1999 Birth-related expression of c-fos, c-jun and substance P mRNAs in the rat brainstem and pia mater: possible relationship to changes in central chemosensitivity. Brain Res Dev Brain Res 112: 255–266
Gauda EB, Bamford OS, Northington FJ 1998 Lack of induction of substance P gene expression by hypoxia and absence of neurokinin 1-receptor mRNAs in the rat carotid body. J Auton Nerv Syst 74: 100–108
Ichikawa H 2002 Innervation of the carotid body: immunohistochemical, denervation, and retrograde tracing studies. Microsc Res Tech 59: 188–195
Finley JC, Katz DM 1992 The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res 572: 108–116
Fidone SJ, Gonzalez C, Dinger BG, Hanson GR 1988 Mechanisms of chemotransmission in the mammalian carotid body. Prog Brain Res 74: 169–179
Naftchi NE, Maker H, Lapin E, Sleis J, Lajtha A, Leeman S 1988 Acute reduction of brain substance P induced by nicotine. Neurochem Res 13: 305–309
Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR 1988 Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res 73: 137–157
Nasrat HA, Al-Hachim GM, Mahmood FA 1986 Perinatal effects of nicotine. Biol Neonate 49: 8–14
Lacoste B, Riad M, Descarries L 2006 Immunocytochemical evidence for the existence of substance P receptor (NK1) in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur J Neurosci 23: 2947–2958
Murrin LC, Ferrer JR, Zeng WY, Haley NJ 1987 Nicotine administration to rats: methodological considerations. Life Sci 40: 1699–1708
Slotkin TA, Orband-Miller L, Queen KL, Whitmore WL, Seidler FJ 1987 Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J Pharmacol Exp Ther 240: 602–611
Slotkin TA, McCook EC, Seidler FJ 1997 Cryptic brain cell injury caused by fetal nicotine exposure is associated with persistent elevations of c-fos protooncogene expression. Brain Res 750: 180–188
Brodin E, Rosen A, Theodorsson E, Jonczyk A, Sandberg BE, Brodin K 1994 Multiple molecular forms of tachykinins in rat spinal cord: a study comparing different extraction methods. Regul Pept 52: 97–110
Brodin E, Lindefors N, Dalsgaard CJ, Theodorsson-Norheim E, Rosell S 1986 Tachykinin multiplicity in rat central nervous system as studied using antisera raised against substance P and neurokinin A. Regul Pept 13: 253–272
Brodin E, Linderoth B, Gazelius B, Ungerstedt U 1987 In vivo release of substance P in cat dorsal horn studied with microdialysis. Neurosci Lett 76: 357–362
Thor KB, Helke CJ 1987 Serotonin- and substance P-containing projections to the nucleus tractus solitarii of the rat. J Comp Neurol 265: 275–293
Thor KB, Helke CJ 1989 Serotonin and substance P colocalization in medullary projections to the nucleus tractus solitarius: dual-colour immunohistochemistry combined with retrograde tracing. J Chem Neuroanat 2: 139–148
Zhang WB, Li JS, Li HM 1991 SP-like immunoreactivity in the primary trigeminal neurons projecting to the nucleus tractus solitarii. Brain Res 558: 87–89
Pfaller K, Arvidsson J 1988 Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J Comp Neurol 268: 91–108
Fuxe K, Siegel RA, Andersson K, Eneroth P, Mascagni F, Agnati LF 1985 Acute continuous exposure to cigarette smoke produces discrete changes in cholecystokinin and substance P levels in the hypothalamus and preoptic area of the male rat. Acta Physiol Scand 125: 437–443
Schneider DA, Galligan JJ 2000 Presynaptic nicotinic acetylcholine receptors in the myenteric plexus of guinea pig intestine. Am J Physiol Gastrointest Liver Physiol 279: G528–G535
Bamford OS, Carroll JL 1999 Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol 117: 29–40
Schalling M, Franco-Cereceda A, Hokfelt T, Persson H, Lundberg JM 1988 Increased neuropeptide Y messenger RNA and peptide in sympathetic ganglia after reserpine pretreatment. Eur J Pharmacol 156: 419–420
Kalia M 1981 Neurohistochemical methods in tracing central respiratory mechanisms. Fed Proc 40: 2365–2371
Ljungdahl A, Hokfelt T, Nilsson G 1978 Distribution of substance P-like immunoreactivity in the central nervous system of the rat—I. Cell bodies and nerve terminals. Neuroscience 3: 861–943
Kalia M, Fuxe K, Hokfelt T, Johansson O, Lang R, Ganten D, Cuello C, Terenius L 1984 Distribution of neuropeptide immunoreactive nerve terminals within the subnuclei of the nucleus of the tractus solitarius of the rat. J Comp Neurol 222: 409–444
Cuello AC, Del Fiacco M, Paxinos G 1978 The central and peripheral ends of the substance P-containing sensory neurones in the rat trigeminal system. Brain Res 152: 499–500
Helke CJ, O'Donohue TL, Jacobowitz DM 1980 Substance P as a baro- and chemoreceptor afferent neurotransmitter: immunocytochemical and neurochemical evidence in the rat. Peptides 1: 1–9
Hokfelt T, Ljungdahl A, Steinbusch H, Verhofstad A, Nilsson G, Brodin E, Pernow B, Goldstein M 1978 Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience 3: 517–538
Damaj MI, Meyer EM, Martin BR 2000 The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology 39: 2785–2791
Molinero MT, Del Rio J 1987 Substance P, nicotinic acetylcholine receptors and antinociception in the rat. Neuropharmacology 26: 1715–1720
Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A 1997 Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther 280: 1117–1136
Andoh T, Itoh H, Watanabe I, Sasaki T, Higashi T 2001 Mechanisms of modulation of neuronal nicotinic receptors by substance P and OAG. Am J Physiol Cell Physiol 281: C1871–C1880
Zhou XF, Marley PD, Livett BG 1991 Substance P modulates the time course of nicotinic but not muscarinic catecholamine secretion from perfused adrenal glands of rat. Br J Pharmacol 104: 159–165
Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF 1995 Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science 269: 1446–1450
Ozawa Y, Takashima S 2002 Developmental neurotransmitter pathology in the brainstem of sudden infant death syndrome: a review and sleep position. Forensic Sci Int 14: S53–S59
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Swedish MRC (04X-2887 and 5234), Swedish Match, Stiftelsen Frimurarna Barnhuset, Sällskapet Barnavård, Stiftelsen Samariten, Marianne and Marcus Wallenbergs Foundation and an Unrestricted Bristol-Myers Squibb Neuroscience Grant.
Rights and permissions
About this article
Cite this article
Berner, J., Ringstedt, T., Brodin, E. et al. Prenatal Exposure to Nicotine Affects Substance P and Preprotachykinin-A mRNA Levels in Newborn Rat. Pediatr Res 64, 621–624 (2008). https://doi.org/10.1203/PDR.0b013e318186e5f5
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e318186e5f5
This article is cited by
-
Prenatal nicotine exposure decreases the release of dopamine in the medial frontal cortex and induces atomoxetine-responsive neurobehavioral deficits in mice
Psychopharmacology (2017)
-
Evaluation of cognitive behaviors in young offspring of C57BL/6J mice after gestational nicotine exposure during different time-windows
Psychopharmacology (2013)