Abstract
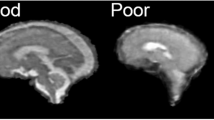
Animal models with complex cortical development are useful for improving our understanding of the wide spectrum of neurodevelopmental challenges facing human preterm infants. MRI techniques can define both cerebral injury and alterations in cerebral development with translation between animal models and the human infant. We hypothesized that the immature ferret would display a similar sequence of brain development [both gray (GM) and white matter (WM)] to that of the preterm human infant. We describe postnatal ferret neurodevelopment with conventional and diffusion MRI. The ferret is born lissencephalic with a thin cortical plate and relatively large ventricles. Cortical folding and WM maturation take place during the first month of life. From the mid-second through the third week of postnatal life, the ferret brain undergoes a similar, though less complex, pattern of maturational changes to those observed in the human brain during the second half of gestation. GM anisotropy decreases rapidly in the first 3 wks of life, followed by an upward surge of surface folding and WM anisotropy over the next 2 wks.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ADC:
-
apparent diffusion coefficients
- E:
-
embryonic day
- GM:
-
gray matter
- P:
-
postnatal day
- RA:
-
relative anisotropy
- SFI:
-
surface folding index
- WM:
-
white matter
References
Moster D, Lie RT, Markestad T 2008 Long-term medical and social consequences of preterm birth. N Engl J Med 359: 262–273
Back SA, Riddle A, Hohimer AR 2006 Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol 21: 582–589
Derrick M, Drobyshevsky A, Ji X, Tan S 2007 A model of cerebral palsy from fetal hypoxia-ischemia. Stroke 38: 731–735
Dieni S, Inder T, Yoder B, Briscoe T, Camm E, Egan G, Denton D, Rees S 2004 The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Neuropathol Exp Neurol 63: 1297–1309
Duncan JR, Cock ML, Scheerlinck JP, Westcott KT, McLean C, Harding R, Rees SM 2002 White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res 52: 941–949
Hagberg H, Bona E, Gilland E, Puka-Sundvall M 1997 Hypoxia-ischaemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl 422: 85–88
Kroenke CD, Van Essen DC, Inder TE, Rees S, Bretthorst GL, Neil JJ 2007 Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci 27: 12506–12515
Lodygensky GA, Inder TE, Neil JJ 2008 Application of magnetic resonance imaging in animal models of perinatal hypoxic-ischemic cerebral injury. Int J Dev Neurosci 26: 13–25
Katz LC, Crowley JC 2002 Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci 3: 34–42
Christensson M, Garwicz M 2005 Ontogenesis of within-session locomotor habituation in the open field. Neuroreport 16: 1319–1323
Christensson M, Garwicz M 2005 Time course of postnatal motor development in ferrets: ontogenetic and comparative perspectives. Behav Brain Res 158: 231–242
Neal J, Takahashi M, Silva M, Tiao G, Walsh CA, Sheen VL 2007 Insights into the gyrification of developing ferret brain by magnetic resonance imaging. J Anat 210: 66–77
Martin E, Kikinis R, Zuerrer M, Boesch C, Briner J, Kewitz G, Kaelin P 1988 Developmental stages of human brain: an MR study. J Comput Assist Tomogr 12: 917–922
Hahn EL 1950 Spin Echoes. Phys Rev 80: 580–594
Batchelor PG, Atkinson D, Hill DL, Calamante F, Connelly A 2003 Anisotropic noise propagation in diffusion tensor MRI sampling schemes. Magn Reson Med 49: 1143–1151
Neil JJ, Bretthorst GL 1993 On the use of Bayesian probability theory for analysis of exponential decay data: an example taken from intravoxel incoherent motion experiments. Magn Reson Med 29: 642–647
Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH 2001 An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 8: 443–459
Van Essen DC 2005 A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage 28: 635–662
McSherry GM, Smart IH 1986 Cell production gradients in the developing ferret isocortex. J Anat 144: 1–14
Smart IH, McSherry GM 1986 Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J Anat 147: 27–43
Jackson CA, Peduzzi JD, Hickey TL 1989 Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J Neurosci 9: 1242–1253
Noctor SC, Scholnicoff NJ, Juliano SL 1997 Histogenesis of ferret somatosensory cortex. J Comp Neurol 387: 179–193
McKinstry RC, Mathur A, Miller JH, Ozcan A, Snyder AZ, Schefft GL, Almli CR, Shiran SI, Conturo TE, Neil JJ 2002 Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex 12: 1237–1243
Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ 2005 Abnormal cerebral structure is present at term in premature infants. Pediatrics 115: 286–294
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE 2006 Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355: 685–694
Counsell SJ, Allsop JM, Harrison MC, Larkman DJ, Kennea NL, Kapellou O, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA 2003 Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 112: 1–7
McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR, Shimony JS, Shiran SI, Neil JJ 2002 A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 59: 824–833
Ward P, Counsell S, Allsop J, Cowan F, Shen Y, Edwards D, Rutherford M 2006 Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics 117: e619–e630
Rees S, Stringer M, Just Y, Hooper SB, Harding R 1997 The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Brain Res Dev Brain Res 103: 103–118
Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Huppi PS 2008 Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 18: 1444–1454
Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S 2005 Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 25: 5988–5997
Sun SW, Neil JJ, Song SK 2003 Relative indices of water diffusion anisotropy are equivalent in live and formalin-fixed mouse brains. Magn Reson Med 50: 743–748
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Green Foundation (J.J.N.), National Science Foundation Grant DMS-0540701 (P.V.B.), AAP Marshall Klaus Perinatal Research Grant (A.R.B.).
Rights and permissions
About this article
Cite this article
Barnette, A., Neil, J., Kroenke, C. et al. Characterization of Brain Development in the Ferret via MRI. Pediatr Res 66, 80–84 (2009). https://doi.org/10.1203/PDR.0b013e3181a291d9
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181a291d9
This article is cited by
-
Postnatal refinement of interareal feedforward projections in ferret visual cortex
Brain Structure and Function (2018)
-
Zinc histochemistry reveals circuit refinement and distinguishes visual areas in the developing ferret cerebral cortex
Brain Structure and Function (2013)
-
Histopathologic correlation with diffusion tensor imaging after chronic hypoxia in the immature ferret
Pediatric Research (2012)