Abstract
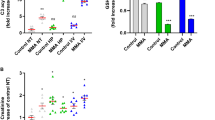
We investigated respiratory chain (RC), tricarboxylic acid cycle (TCA) enzyme activities, and oxidative stress in the tissues of six patients with organic aciduria (OA) presenting various severe complications to further document the role of mitochondrial OXPHOS dysfunction in the development of complications. Two children with propionic acidemia (PA), presenting a severe cardiomyopathy, and four with methylmalonic aciduria (MMA), who developed a neurologic disease (3/4) and renal failure (2/4), were followed. We measured RC and TCA cycle enzyme activity in patient tissues and assessed oxidative metabolism in fibroblasts in vitro. Various RC deficiencies were found in tissues of patients with PA and MMA. TCA cycle enzyme activities were normal when investigated and reactive oxygen species were decreased as well as detoxifying systems activities in the two patients tested. In conclusion, mitochondrial dysfunction was found in all investigated tissues of six patients with organic acidemia presenting with severe complications. Reactive oxygen species production and detoxification were decreased in fibroblast primary cultures. Our results bring further support for a role of secondary respiratory deficiency in the development of late multiorgan complications of these diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MA:
-
methylmalonic acid
- MCM:
-
methylmalonyl-CoA mutase
- MMA:
-
methylmalonyl aciduria
- OA:
-
organic aciduria
- PA:
-
propionic acidemia
- RC:
-
respiratory chain
- TCA:
-
tricarboxylic acid
References
Touati G, Valayannopoulos V, Mention K, de Lonlay P, Jouvet P, Depondt E, Assoun M, Souberbielle JC, Rabier D, Ogier de Baulny H, Saudubray JM 2006 Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J Inherit Metab Dis 29: 288–298
Dionisi-Vici C, Deodato F, Roschinger W, Rhead W, Wilcken B 2006 ‘Classical' organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inherit Metab Dis 29: 383–389
Walter JH, Michalski A, Wilson WM, Leonard JV, Barratt TM, Dillon MJ 1989 Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr 148: 344–348
Kahler SG, Sherwood WG, Woolf D, Lawless ST, Zaritsky A, Bonham J, Taylor CJ, Clarke JT, Durie P, Leonard JV 1994 Pancreatitis in patients with organic acidemias. J Pediatr 124: 239–243
Burlina AB, Dionisi-Vici C, Piovan S, Saponara I, Bartuli A, Sabetta G, Zacchello F 1995 Acute pancreatitis in propionic acidaemia. J Inherit Metab Dis 18: 169–172
Mardach R, Verity MA, Cederbaum SD 2005 Clinical, pathological, and biochemical studies in a patient with propionic acidemia and fatal cardiomyopathy. Mol Genet Metab 85: 286–290
Burlina AP, Manara R, Calderone M, Catuogno S, Burlina AB 2003 Diffusion-weighted imaging in the assessment of neurological damage in patients with methylmalonic aciduria. J Inherit Metab Dis 26: 417–422
Chandler RJ, Zerfas PM, Shanske S, Sloan J, Hoffmann V, Dimauro S, Venditti CP 2009 Mitochondrial dysfunction in mut methylmalonic acidemia. FASEB J 23: 1252–1261
Schwab MA, Sauer SW, Okun JG, Nijtmans LG, Rodenburg RJ, van den Heuvel LP, Drose S, Brandt U, Hoffmann GF, Ter Laak H, Kolker S, Smeitink JA 2006 Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins. Biochem J 398: 107–112
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray J, Munnich A 1994 Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228: 35–51
Blomstrand E, Radegran G, Saltin B 1997 Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J Physiol 501: 455–460
Alexandre J, Batteux F, Nicco C, Chereau C, Laurent A, Guillevin L, Weill B, Goldwasser F 2006 Accumulation of hydrogen peroxide is an early and crucial step for paclitaxel-induced cancer cell death both in vitro and in vivo. Int J Cancer 119: 41–48
Aebi H 1984 Catalase in vitro. Methods Enzymol 105: 121–126
Beauchamp C, Fridovich I 1971 Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44: 276–287
Cosson MA, Touati G, Lacaille F, Valayannnopoulos V, Guyot C, Guest G, Verkarre V, Chrétien D, Rabier D, Munnich A, Benoist JF, de Keyzer Y, Niaudet P, de Lonlay P 2008 Liver hepatocarcinoma and multiple OXPHOS deficiency in the follow-up of a patient with methylmalonic aciduria. Mol Genet Metab 95: 107–109
Acquaviva C, Benoist JF, Pereira S, Callebaut I, Koskas T, Porquet D, Elion J 2005 Molecular basis of methylmalonyl-CoA mutase apoenzyme defect in 40 European patients affected by mut(o) and mut- forms of methylmalonic acidemia: identification of 29 novel mutations in the MUT gene. Hum Mutat 25: 167–176
Cheema-Dhadli S, Leznoff CC, Halperin ML 1975 Effect of 2-methylcitrate on citrate metabolism: implications for the management of patients with propionic acidemia and methylmalonic aciduria. Pediatr Res 9: 905–908
Brusque AM, Borba Rosa R, Schuck PF, Dalcin KB, Ribeiro CA, Silva CG, Wannmacher CM, Dutra-Filho CS, Wyse AT, Briones P, Wajner M 2002 Inhibition of the mitochondrial respiratory chain complex activities in rat cerebral cortex by methylmalonic acid. Neurochem Int 40: 593–601
Okun JG, Horster F, Farkas LM, Feyh P, Hinz A, Sauer S, Hoffmann GF, Unsicker K, Mayatepek E, Kolker S 2002 Neurodegeneration in methylmalonic aciduria involves inhibition of complex II and the tricarboxylic acid cycle, and synergistically acting excitotoxicity. J Biol Chem 277: 14674–14680
Haas RH, Marsden DL, Capistrano-Estrada S, Hamilton R, Grafe MR, Wong W, Nyhan WL 1995 Acute basal ganglia infarction in propionic acidemia. J Child Neurol 10: 18–22
Schmitt CP, Mehls O, Trefz FK, Horster F, Weber TL, Kolker S 2004 Reversible end-stage renal disease in an adolescent patient with methylmalonic aciduria. Pediatr Nephrol 19: 1182–1184
Mirandola SR, Melo DR, Schuck PF, Ferreira GC, Wajner M, Castilho RF 2008 Methylmalonate inhibits succinate-supported oxygen consumption by interfering with mitochondrial succinate uptake. J Inherit Metab Dis 31: 44–54
Morath MA, Okun JG, Muller IB, Sauer SW, Horster F, Hoffmann GF, Kolker S 2008 Neurodegeneration and chronic renal failure in methylmalonic aciduria—a pathophysiological approach. J Inherit Metab Dis 31: 35–43
Burckhardt BC, Burckhardt G 2003 Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol 146: 95–158
Brunengraber H, Roe CR 2006 Anaplerotic molecules: current and future. J Inherit Metab Dis 29: 327–331
Treacy E, Arbour L, Chessex P, Graham G, Kasprzak L, Casey K, Bell L, Mamer O, Scriver CR 1996 Glutathione deficiency as a complication of methylmalonic acidemia: Response to high doses of ascorbate. J Pediatr 129: 445–448
Richard E, Alvarez-Barrientos A, Perez B, Desviat LR, Ugarte M 2007 Methylmalonic acidaemia leads to increased production of reactive oxygen species and induction of apoptosis through the mitochondrial/caspase pathway. J Pathol 213: 453–461
Wajner M, Coelho JC 1997 Neurological dysfunction in methylmalonic acidaemia is probably related to the inhibitory effect of methylmalonate on brain energy production. J Inherit Metab Dis 20: 761–768
Carrozzo R, Dionisi-Vici C, Steuerwald U, Lucioli S, Deodato F, Di Giandomenico S, Bertini E, Franke B, Kluijtmans LA, Meschini MC, Rizzo C, Piemonte F, Rodenburg R, Santer R, Santorelli FM, van Rooij A, Vermunt-de Koning D, Morava E, Wevers RA 2007 SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain 130: 862–874
Ostergaard E, Christensen E, Kristensen E, Mogensen B, Duno M, Shoubridge EA, Wibrand F 2007 Deficiency of the alpha subunit of succinate-coenzyme. A ligase causes fatal infantile lactic acidosis with mitochondrial DNA depletion. Am J Hum Genet 81: 383–387
Ostergaard E, Hansen FJ, Sorensen N, Duno M, Vissing J, Larsen PL, Faeroe O, Thorgrimsson S, Wibrand F, Christensen E, Schwartz M 2007 Mitochondrial encephalomyopathy with elevated methylmalonic acid is caused by SUCLA2 mutations. Brain 130: 853–861
Elpeleg O, Miller C, Hershkovitz E, Bitner-Glindzicz M, Bondi-Rubinstein G, Rahman S, Pagnamenta A, Eshhar S, Saada A 2005 Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am J Hum Genet 76: 1081–1086
Barshes NR, Vanatta JM, Patel AJ, Carter BA, O'Mahony CA, Karpen SJ, Goss JA 2006 Evaluation and management of patients with propionic acidemia undergoing liver transplantation: a comprehensive review. Pediatr Transplant 10: 773–781
Kaplan P, Ficicioglu C, Mazur AT, Palmieri MJ, Berry GT 2006 Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol Genet Metab 88: 322–326
Lubrano R, Elli M, Rossi M, Travasso E, Raggi C, Barsotti P, Carducci C, Berloco P 2007 Renal transplant in methylmalonic acidemia: could it be the best option? Report on a case at 10 years and review of the literature. Pediatr Nephrol 22: 1209–1214
Nagarajan S, Enns GM, Millan MT, Winter S, Sarwal MM 2005 Management of methylmalonic acidaemia by combined liver-kidney transplantation. J Inherit Metab Dis 28: 517–524
Nyhan WL, Gargus JJ, Boyle K, Selby R, Koch R 2002 Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur J Pediatr 161: 377–379
Kolker S, Okun JG 2005 Methylmalonic acid–an endogenous toxin?. Cell Mol Life Sci 62: 621–624
Mochel F, DeLonlay P, Touati G, Brunengraber H, Kinman RP, Rabier D, Roe CR, Saudubray JM 2005 Pyruvate carboxylase deficiency: clinical and biochemical response to anaplerotic diet therapy. Mol Genet Metab 84: 305–312
Acknowledgements
We thank JM Saudubray, N Boddaert, I Desguerre, B Chabrol, and MA Cosson for their contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Fondation Lejeune and Association Française contre la Myopathie (subvention No. 13387).
Rights and permissions
About this article
Cite this article
de Keyzer, Y., Valayannopoulos, V., Benoist, JF. et al. Multiple OXPHOS Deficiency in the Liver, Kidney, Heart, and Skeletal Muscle of Patients With Methylmalonic Aciduria and Propionic Aciduria. Pediatr Res 66, 91–95 (2009). https://doi.org/10.1203/PDR.0b013e3181a7c270
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181a7c270
This article is cited by
-
Cardiac Metabolism, Reprogramming, and Diseases
Journal of Cardiovascular Translational Research (2024)
-
Methylmalonic Acid Impairs Cell Respiration and Glutamate Uptake in C6 Rat Glioma Cells: Implications for Methylmalonic Acidemia
Cellular and Molecular Neurobiology (2023)
-
Disruption of mitochondrial functions involving mitochondrial permeability transition pore opening caused by maleic acid in rat kidney
Journal of Bioenergetics and Biomembranes (2022)
-
Neurological manifestations of organic acidurias
Nature Reviews Neurology (2019)
-
Experimental evidence that maleic acid markedly compromises glutamate oxidation through inhibition of glutamate dehydrogenase and α-ketoglutarate dehydrogenase activities in kidney of developing rats
Molecular and Cellular Biochemistry (2019)