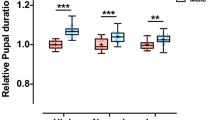
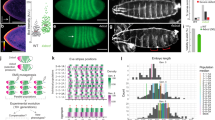
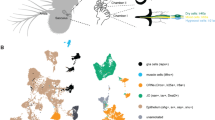
Abstract
We have previously discovered that the adult Drosophila melanogaster is tolerant to a low O2 environment, withstanding hours of total O2 deprivation without showing any evidence of cell injury. Subsequently, our laboratory embarked on the study of hypoxia tolerance using a mutagenesis and overexpression screens to begin to investigate loss-of-function or gain-of-function phenotypes. Both have given us promising results and, in this article, we detail some of the interesting results. Furthermore, several years ago, we have also started an experimental “Darwinian” selection to generate a fly strain that can perpetuate through all of its life cycle stages in hypoxic environments. Through microarrays and bioinformatic analyses, we have obtained genes (e.g. Notch pathway genes) that play an important role in hypoxia resistance. In addition, we also detail a proof of principle that Drosophila genes that are beneficial in fly resistance to hypoxia can also be as well in mammalian cells. We believe that the mechanisms that we are uncovering in Drosophila will allow us to gain insight regarding susceptibility and tolerance to low O2 and will therefore pave the way to develop better therapies for ailments that afflict humans as a consequence of low O2 delivery or low blood O2 levels.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AF:
-
hypoxia-adapted/selected flies
- CH:
-
constant hypoxia
- dADAR:
-
pre-mRNA adenosine deaminase
- dMRP4:
-
Drosophila homolog of the human multidrug resistance protein 4
- IH:
-
intermittent hypoxia
- NF:
-
naïve control flies
References
Haddad GG, Jiang C 1993 Mechanisms of anoxia-induced depolarization in brainstem neurons: in vitro current and voltage clamp studies in the adult rat. Brain Res 625: 261–268
Banasiak KJ, Haddad GG 1998 Hypoxia-induced apoptosis: effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res 797: 295–304
Banasiak KJ, Cronin T, Haddad GG 1999 bcl-2 prolongs neuronal survival during hypoxia-induced apoptosis. Brain Res Mol Brain Res 72: 214–225
Banasiak KJ, Xia Y, Haddad GG 2000 Mechanisms underlying hypoxia-induced neuronal apoptosis. Prog Neurobiol 62: 215–249
Bier E 2005 Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet 6: 9–23
Farahani R, Haddad GG 2003 Understanding the molecular responses to hypoxia using Drosophila as a genetic model. Respir Physiol Neurobiol 135: 221–229
Haddad GG 2006 Tolerance to low O2: lessons from invertebrate genetic models. Exp Physiol 91: 277–282
Bodmer R 1993 The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 118: 719–729
Azpiazu N, Frasch M 1993 tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev 7: 1325–1340
Bier E, Bodmer R 2004 Drosophila, an emerging model for cardiac disease. Gene 342: 1–11
Caudy AA, Myers M, Hannon GJ, Hammond SM 2002 Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev 16: 2491–2496
Ishizuka A, Siomi MC, Siomi H 2002 A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev 16: 2497–2508
Cauchi RJ, van den Heuvel M 2006 The fly as a model for neurodegenerative diseases: is it worth the jump?. Neurodegener Dis 3: 338–356
Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST 2008 Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol 4: 256–263
Douglas RM, Haddad GG 2003 Genetic models in applied physiology: invited review: effect of oxygen deprivation on cell cycle activity: a profile of delay and arrest. J Appl Physiol 94: 2068–2083; discussion 2084
Haddad GG, Sun Y, Wyman RJ, Xu T 1997 Genetic basis of tolerance to O2 deprivation in Drosophila melanogaster. Proc Natl Acad Sci USA 94: 10809–10812
Haddad GG, Wyman RJ, Mohsenin A, Sun Y, Krishnan SN 1997 Behavioral and Electrophysiologic Responses of Drosophila melanogaster to Prolonged Periods of Anoxia. J Insect Physiol 43: 203–210
Chen L, Rio DC, Haddad GG, Ma E 2004 Regulatory role of dADAR in ROS metabolism in Drosophila CNS. Brain Res Mol Brain Res 131: 93–100
Chen Q, Behar KL, Xu T, Fan C, Haddad GG 2003 Expression of Drosophila trehalose-phosphate synthase in HEK-293 cells increases hypoxia tolerance. J Biol Chem 278: 49113–49118
Chen Q, Haddad GG 2004 Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol 207: 3125–3129
Chen Q, Ma E, Behar KL, Xu T, Haddad GG 2002 Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster. J Biol Chem 277: 3274–3279
Huang H, Haddad GG 2007 Drosophila dMRP4 regulates responsiveness to O2 deprivation and development under hypoxia. Physiol Genomics 29: 260–266
Ma E, Gu XQ, Wu X, Xu T, Haddad GG 2001 Mutation in pre-mRNA adenosine deaminase markedly attenuates neuronal tolerance to O2 deprivation in Drosophila melanogaster. J Clin Invest 107: 685–693
Xia S, Yang J, Su Y, Qian J, Ma E, Haddad GG 2005 Identification of new targets of Drosophila pre-mRNA adenosine deaminase. Physiol Genomics 20: 195–202
Zhou D, Xue J, Chen J, Morcillo P, Lambert JD, White KP, Haddad GG 2007 Experimental selection for Drosophila survival in extremely low O2 environment. PLoS One 2: e490
Zhou D, Xue J, Lai JC, Schork NJ, White KP, Haddad GG 2008 Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet 4: e1000221
Ma E, Xu T, Haddad GG 1999 Gene regulation by O2 deprivation: an anoxia-regulated novel gene in Drosophila melanogaster. Brain Res Mol Brain Res 63: 217–224
Ma E, Tucker MC, Chen Q, Haddad GG 2002 Developmental expression and enzymatic activity of pre-mRNA deaminase in Drosophila melanogaster. Brain Res Mol Brain Res 102: 100–104
Hochachka PW 1986 Defense strategies against hypoxia and hypothermia. Science 231: 234–241
Hochachka PW, Clark CM, Brown WD, Stanley C, Stone CK, Nickles RJ, Zhu GG, Allen PS, Holden JE 1994 The brain at high altitude: hypometabolism as a defense against chronic hypoxia?. J Cereb Blood Flow Metab 14: 671–679
Hochachka PW, Dunn JF 1983 Metabolic arrest: the most effective means of protecting tissues against hypoxia. Prog Clin Biol Res 136: 297–309
Iacobas DA, Fan C, Iacobas S, Spray DC, Haddad GG 2006 Transcriptomic changes in developing kidney exposed to chronic hypoxia. Biochem Biophys Res Commun 349: 329–338
Zhou D, Wang J, Zapala MA, Xue J, Schork NJ, Haddad GG 2008 Gene expression in mouse brain following chronic hypoxia: role of sarcospan in glial cell death. Physiol Genomics 32: 370–379
Farahani R, Kanaan A, Gavrialov O, Brunnert S, Douglas RM, Morcillo P, Haddad GG 2008 Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr Pulmonol 43: 20–28
Iacobas DA, Fan C, Iacobas S, Haddad GG 2008 Integrated transcriptomic response to cardiac chronic hypoxia: translation regulators and response to stress in cell survival. Funct Integr Genomics 8: 265–275
Fan C, Iacobas DA, Zhou D, Chen Q, Lai JK, Gavrialov O, Haddad GG 2005 Gene expression and phenotypic characterization of mouse heart after chronic constant or intermittent hypoxia. Physiol Genomics 22: 292–307
Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG 2006 Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol 290: R1105–R1114
Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP 2007 Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol 292: R1254–R1259
Chandel NS, Budinger GR 2007 The cellular basis for diverse responses to oxygen. Free Radic Biol Med 42: 165–174
Dewhirst MW, Cao Y, Moeller B 2008 Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8: 425–437
Haddad GG 1997 K+ ions and channels: mechanisms of sensing O2 deprivation in central neurons. Kidney Int 51: 467–472
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Grants NIH P01 HD 32573 and NIH R01 NS 037756 from the National Institutes of Health (to G.G.H) and the Grant AHA 0835188N from the American Heart Association (to D.Z.).
The Role of Oxygen in Health and Disease - A Series of Reviews
This is the fifth and last article in the series of reviews focusing on the role that oxygen plays in health and disease. As a proof of principle, Drs. Zhou, Visk and Haddad have described Drosophila genes, beneficial in developing resistance to hypoxia, to provide insight regarding susceptibility and tolerance to low oxygen. This information will pave the way towards developing improved therapies for mammalian species to combat the consequences of low oxygen delviery and low blood oxygen levels.
Sherin U. Devaskar, M.D.
Editor in Chief
Rights and permissions
About this article
Cite this article
Zhou, D., Visk, D. & Haddad, G. Drosophila, a Golden Bug, for the Dissection of the Genetic Basis of Tolerance and Susceptibility to Hypoxia. Pediatr Res 66, 239–247 (2009). https://doi.org/10.1203/PDR.0b013e3181b27275
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181b27275
This article is cited by
-
Pgant4 and Tango1 Mediate Anoxia and Reoxygenation Injury
Neuroscience Bulletin (2020)
-
Proteome-wide analysis of Anopheles culicifacies mosquito midgut: new insights into the mechanism of refractoriness
BMC Genomics (2018)
-
An Efficient and Reliable Assay for Investigating the Effects of Hypoxia/Anoxia on Drosophila
Neuroscience Bulletin (2018)