Abstract
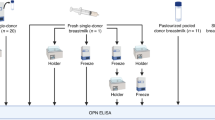
Pasteurizing donor human milk inactivates bacteria that may be of concern to the preterm infant. However, current practice for Holder Pasteurization (62.5°C for 30 min) is detrimental to the bioactivity of human milk. An experimental pasteurizer was used to determine the maximum temperature at which 90% of secretory IgA, lysozyme, and lactoferrin were retained and whether this temperature was capable of inactivating five common bacterial contaminants. The retention of these proteins was also compared using a commercially available bottle immersion or holding chamber system. After pasteurization at 62.5°C for 30 min, the retention across all three systems was 72.3 ± 3.6%, 21.8 ± 3.3%, and 39.4 ± 11.5% for sIgA, lactoferrin, and lysozyme, respectively (n = 22). The retention of all three proteins was at least 90% when human milk was pasteurized at 57°C for 30 min, and this temperature was also effective at removing 99.9% of all inoculated bacterial species. In addition, human milk that was pasteurized in the experimental system had a significantly higher proportion of lysozyme compared with samples pasteurized in the bottle immersion system. These findings suggest that optimizing pasteurization temperature and improving pasteurizer design enhances the quality of pasteurized donor human milk.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CFU/ml:
-
colony-forming units/milliliter
- DHM:
-
donor human milk
- MOM:
-
mother's own milk
- PDHM:
-
pasteurized donor human milk
- sIgA:
-
secretory immunoglobulin A
References
Wight NE 2001 Donor human milk for preterm infants. J Perinatol 21: 249–254
Hartmann BT, Pang WW, Keil AD, Hartmann PE, Simmer K 2007 Best practice guidelines for the operation of a donor human milk bank in an Australian NICU. Early Hum Dev 83: 667–673
Human Milk Banking Association of North America 1995 Guidelines for the establishment and operation of a donor human milk bank. HMBANA, West Hartford, CT pp 1–28
Balmer SE, Wiliams AF 1995 Guidelines for the establishment and operation of human milk banks in the UK. Arch Dis Child 73: 481–482
Enright JB, Sadler WW, Thomas RC 1957 Thermal inactivation of Coxiella burnetii and its relation to pasteurization of milk. Public Health Monogr 47: 1–30
Bylund G 2003 Dairy processing handbook. Tetra Pak Processing Systems AB, Lund, Sweden 451–453
Smelt JP, Hellemons JC, Wouters PC, van Gerwen SJ 2002 Physiological and mathematical aspects in setting criteria for decontamination of foods by physical means. Int J Food Microbiol 78: 57–77
Björkstén B, Burman LG, De Château P, Fredrikzon B, Gothefors L, Hernell O 1980 Collecting and banking human milk: to heat or not to heat?. BMJ 281: 765–769
Wills ME, Han VE, Harris DA, Baum JD 1982 Short-time low-temperature pasteurisation of human milk. Early Hum Dev 7: 71–80
Lawrence RA 2001 Milk banking: the influence of storage procedures and subsequent processing on immunologic components of human milk. Adv Nutr Res 10: 389–404
Lucas A, Cole TJ 1990 Breast milk and neonatal necrotizing enterocolitis. Lancet 336: 1519–1523
Prentice A, Ewing G, Roberts SB, Lucas A, MacCarthy A, Jarjou LM, Whitehead RG 1987 The nutritional role of breast-milk IgA and lactoferrin. Acta Paediatr Scand 76: 592–598
Monod J 1949 The growth of bacterial cultures. Annu Rev Microbiol 3: 371–394
Lonnerdal B 2003 Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr 77: 1537S–1543S
Ford JE, Law BA, Marshall VM, Reiter B 1977 Influence of the heat treatment of human milk on some of its protective constituents. J Pediatr 90: 29–35
Hamprecht K, Maschmann J, Müller D, Dietz K, Besenthal I, Goelz R, Middeldorp JM, Speer CP, Jahn G 2004 Cytomegalovirus (CMV) inactivation in breast milk: reassessment of pasteurization and freeze-thawing. Pediatr Res 56: 529–535
Lequin RM 2005 Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem 51: 2415–2418
Jones CL, Jennison RF, D'Souza SW 1979 Bacterial contamination of expressed breast milk. BMJ 2: 1320–1322
David JR, Graves RH 1996 Aseptic Processing and Packaging of Food: A Food Industry Perspective. CRC Press, Boca Raton pp 3–21
Ball CO 1943 Short-time pasteurization of milk. Ind Eng Chem 35: 71–84
Acknowledgements
The authors are grateful for the assistance of Mr. Lukas Christen and Mr. Beat Widmer (Carag AG, Switzerland) for providing the technical diagrams of the experimental pasteurizer and to the generosity of the human milk donors that made this research possible.
Author information
Authors and Affiliations
Additional information
Supported by the Rotary Clubs of Thornlie and Belmont (Western Australia), The Women and Infants Research Foundation, The Perron Charitable Trust, and Medela AG (Switzerland).
Rights and permissions
About this article
Cite this article
Czank, C., Prime, D., Hartmann, B. et al. Retention of the Immunological Proteins of Pasteurized Human Milk in Relation to Pasteurizer Design and Practice. Pediatr Res 66, 374–379 (2009). https://doi.org/10.1203/PDR.0b013e3181b4554a
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181b4554a
This article is cited by
-
Impact of time-temperature combinations on the anti-Cytomegalovirus activity and biological components of human milk
Pediatric Research (2023)
-
A Comprehensive Analysis of Maternal and Newborn Disease and Related Control for COVID-19
SN Comprehensive Clinical Medicine (2021)
-
Improving Quality of Banked Milk: Utility of Dornic Acid Test
The Indian Journal of Pediatrics (2018)
-
Empfehlungen zur Förderung von Frauenmilchbanken in Deutschland, Österreich und der Schweiz (D-A-CH-Raum)
Monatsschrift Kinderheilkunde (2018)
-
Effect of high pressure and heat treatments on IgA immunoreactivity and lysozyme activity in human milk
European Food Research and Technology (2016)