Abstract
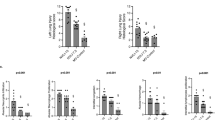
Herein, we determined the contribution of mechanical ventilation, hyperoxia and inflammation, individually or combined, to the cytokine/chemokine response of the neonatal lung. Eight-day-old rats were ventilated for 8 h with low (∼3.5 mL/kg), moderate (∼12.5 mL/kg), or high (∼25 mL/kg) tidal volumes (VT) and the cytokine/chemokine response was measured. Next, we tested whether low-VT ventilation with 50% oxygen or a preexisting inflammation induced by lipopolysaccharide (LPS) would modify this response. High-, moderate-, and low-VT ventilation significantly elevated CXCL-2 and IL-6 mRNA levels. Low-VT ventilation with 50% oxygen significantly increased IL-6 and CXCL-2 expression versus low-VT ventilation alone. LPS pretreatment combined with low-VT ventilation with 50% oxygen amplified IL-6 mRNA expression when compared with low VT alone or low VT + 50% O2 treatment. In contrast, low VT up-regulated CXCL-2 levels were reduced to nonventilated levels when LPS-treated newborn rats were ventilated with 50% oxygen. Thus, low-VT ventilation triggers the expression of acute phase cytokines and CXC chemokines in newborn rat lung, which is amplified by oxygen but not by a preexisting inflammation. Depending on the individual cytokine or chemokine, the combination of both oxygen and inflammation intensifies or abrogates the low VT-induced inflammatory response.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- VT:
-
tidal volume
- BPD:
-
bronchopulmonary dysplasia
- BALF:
-
bronchoalveolar lavage
- HFOV:
-
high frequency oscillatory ventilation
- LPS:
-
lipopolysaccharide
- NV:
-
non-ventilated
- MV:
-
mechanical ventilation
- LVT:
-
MV with low tidal volume
- MVT:
-
MV with moderate tidal volume
- HVT:
-
MV with high tidal volume
- LVT + LPS:
-
LVT after exposure to LPS
- LVT + O2:
-
LVT and 50% oxygen
- LVT + LPS/O2:
-
LVT and 50% oxygen after exposure to LPS
- PEEP:
-
positive end expiratory pressure
- MPO:
-
myeloperoxidase
- CXCL:
-
chemokine (C-X-C motif) ligand
References
Speer CP 2006 Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med 11: 354–362
Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, Janisse J, Mazor M 1998 Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 78: 5–10
Groneck P, Gotze-Speer B, Oppermann M, Eiffert H, Speer CP 1994 Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93: 712–718
Yoder BA, Siler-Khodr T, Winter VT, Coalson JJ 2000 High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. Am J Respir Crit Care Med 162: 1867–1876
Imai Y, Nakagawa S, Ito Y, Kawano T, Slutsky AS, Miyasaka K 2001 Comparison of lung protection strategies using conventional and high-frequency oscillatory ventilation. J Appl Physiol 91: 1836–1844
Altemeier WA, Matute-Bello G, Frevert CW, Kawata Y, Kajikawa O, Martin TR, Glenny RW 2004 Mechanical ventilation with moderate tidal volumes synergistically increases lung cytokine response to systemic endotoxin. Am J Physiol Lung Cell Mol Physiol 287: L533–L542
Roth-Kleiner M, Ridsdale R, Cao L, Kuliszewski M, Tseu I, McKerlie C, Post M 2007 Lipopolysaccharide exposure modifies high tidal volume ventilation-induced proinflammatory mediator expression in newborn rat lungs. Pediatr Res 61: 191–196
Bonikos DS, Bensch KG, Northway WH Jr, Edwards DK 1976 Bronchopulmonary dysplasia: the pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum Pathol 7: 643–666
Jankov RP, Johnstone L, Luo X, Robinson BH, Tanswell AK 2003 Macrophages as a major source of oxygen radicals in the hyperoxic newborn rat lung. Free Radic Biol Med 35: 200–209
Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, Tanswell AK 2004 Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 170: 1188–1196
Davis JM, Dickerson B, Metlay L, Penney DP 1991 Differential effects of oxygen and barotrauma on lung injury in the neonatal piglet. Pediatr Pulmonol 10: 157–163
Delemos RA, Coalson JJ, Gerstmann DR, Kuehl TJ, Null DM Jr 1987 Oxygen toxicity in the premature baboon with hyaline membrane disease. Am Rev Respir Dis 136: 677–682
Copland IB, Kavanagh BP, Engelberts D, McKerlie C, Belik J, Post M 2003 Early changes in lung gene expression due to high tidal volume. Am J Respir Crit Care Med 168: 1051–1059
Copland IB, Martinez F, Kavanagh BP, Engelberts D, McKerlie C, Belik J, Post M 2004 High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med 169: 739–748
Liu Q, Lowry TF, Wong-Riley MT 2006 Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J Physiol 577: 957–970
Capoluongo E, Vento G, Santonocito C, Matassa PG, Vaccarella C, Giardina B, Romagnoli C, Zuppi C, Ameglio F 2005 Comparison of serum levels of seven cytokines in premature newborns undergoing different ventilatory procedures: high frequency oscillatory ventilation or synchronized intermittent mandatory ventilation. Eur Cytokine Netw 16: 199–205
Vento G, Matassa PG, Ameglio F, Capoluongo E, Zecca E, Tortorolo L, Martelli M, Romagnoli C 2005 HFOV in premature neonates: effects on pulmonary mechanics and epithelial lining fluid cytokines. A randomized controlled trial. Intensive Care Med 31: 463–470
Thome U, Gotze-Speer B, Speer CP, Pohlandt F 1998 Comparison of pulmonary inflammatory mediators in preterm infants treated with intermittent positive pressure ventilation or high frequency oscillatory ventilation. Pediatr Res 44: 330–337
Vaneker M, Halbertsma FJ, van Egmond J, Netea MG, Dijkman HB, Snijdelaar DG, Joosten LA, van der Hoeven JG, Scheffer GJ 2007 Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology 107: 419–426
Yamamoto H, Teramoto H, Uetani K, Igawa K, Shimizu E 2002 Cyclic stretch upregulates interleukin-8 and transforming growth factor-beta1 production through a protein kinase C-dependent pathway in alveolar epithelial cells. Respirology 7: 103–109
Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD 1999 Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol 277: L167–L173
Sibille Y, Reynolds HY 1990 Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 141: 471–501
Jobe AH, Ikegami M 1998 Mechanisms initiating lung injury in the preterm. Early Hum Dev 53: 81–94
Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM 2002 Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 110: 1703–1716
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS 1997 Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99: 944–952
Gurkan OU, O'Donnell C, Brower R, Ruckdeschel E, Becker PM 2003 Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol 285: L710–L718
Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA 2000 Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 22: 535–542
Choo-Wing R, Nedrelow JH, Homer RJ, Elias JA, Bhandari V 2007 Developmental differences in the responses of IL-6 and IL-13 transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 293: L142–L150
Natarajan S, Kim J, Remick DG 2010 Chronic pulmonary LPS tolerance induces selective immunosuppression while maintaining the neutrophilic response. Shock 33: 162–169
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by operating grants (MOP-15272) from the Canadian Institute of Health Research and the Sophia Children's Hospital Fund (SSWO) and infrastructure grants (CCURE, CSCCD) from the Canadian Foundation for Innovation. M.P. holds a Canadian Research Chair in Fetal, Neonatal and Maternal Health.
Rights and permissions
About this article
Cite this article
Kroon, A., Wang, J., Huang, Z. et al. Inflammatory Response to Oxygen and Endotoxin in Newborn Rat Lung Ventilated With Low Tidal Volume. Pediatr Res 68, 63–69 (2010). https://doi.org/10.1203/PDR.0b013e3181e17caa
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181e17caa
This article is cited by
-
Perinatal origins of bronchopulmonary dysplasia—deciphering normal and impaired lung development cell by cell
Molecular and Cellular Pediatrics (2023)
-
Association of immune cell recruitment and BPD development
Molecular and Cellular Pediatrics (2022)
-
The association between clinical and biochemical characteristics of late-onset sepsis and bronchopulmonary dysplasia in preterm infants
European Journal of Pediatrics (2021)
-
Preterm birth and sustained inflammation: consequences for the neonate
Seminars in Immunopathology (2020)
-
Systemic pro-inflammatory cytokine status following therapeutic hypothermia in a piglet hypoxia-ischemia model
Journal of Neuroinflammation (2017)