Abstract
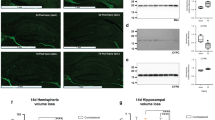
Biomarkers are required for efficient trials of neuroprotective interventions after perinatal asphyxia. This study aimed to determine whether diffusion tensor imaging (DTI) analyzed by tract-based spatial statistics (TBSS) may be a suitable biomarker of disease and treatment effects after perinatal asphyxia in small groups of patients. We performed TBSS from DTI obtained at 3 T from eight healthy control infants, 10 untreated and 10 hypothermia-treated infants with neonatal encephalopathy. Median (range) postnatal age at scan was 1 d (1–21) in the healthy infants, 6 d (4–20) in the cooled, and 7 d (4–18) in noncooled infants. Compared with the control group, fractional anisotropy (FA) was significantly reduced not only in several white matter tracts in the noncooled infants but also in the internal capsule in the cooled group. Noncooled infants had significantly lower FA than the cooled treated infants, indicating more extensive damage, in the anterior and posterior limbs of the internal capsule, the corpus callosum, and optic radiations. We conclude that perinatal hypoxic ischemic encephalopathy is associated with widespread white matter abnormalities that are reduced by moderate hypothermia. DTI analyzed by TBSS detects this treatment effect and is therefore a qualified biomarker for the early evaluation of neuroprotective interventions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DTI:
-
diffusion tensor imaging
- FA:
-
fractional anisotropy
- MR:
-
magnetic resonance
- ROI:
-
region of interest
- TBSS:
-
tract based spatial statistics
References
Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P 2005 The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr 94: 287–294
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ 2005 Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365: 663–670
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH 2005 Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353: 1574–1584
Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P 2009 Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 361: 1349–1358
Ma D, Hossain M, Chow A, Arshad M, Battson RM, Sanders RD, Mehmet H, Edwards AD, Franks NP, Maze M 2005 Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Ann Neurol 58: 182–193
Jouvet P, Cowan FM, Cox P, Lazda E, Rutherford MA, Wigglesworth J, Mehmet H, Edwards AD 1999 Reproducibility and accuracy of MR imaging of the brain after severe birth asphyxia. AJNR Am J Neuroradiol 20: 1343–1348
Rutherford MA, Pennock JM, Counsell SJ, Mercuri E, Cowan FM, Dubowitz LM, Edwards AD 1998 Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 102: 323–328
Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S, Cowan F 2006 Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol 36: 582–592
Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, Meiners LC, Dubowitz LM, de Vries LS 2003 Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 361: 736–742
Chao CP, Zaleski CG, Patton AC 2006 Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings. Radiographics 26: S159–S172
Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D 2010 Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 9: 39–45
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G 1996 Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648
Basser PJ, Pierpaoli C 1996 Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111: 209–219
Ward P, Counsell S, Allsop J, Cowan F, Shen Y, Edwards D, Rutherford M 2006 Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics 117: e619–e630
Hunt RW, Neil JJ, Coleman LT, Kean MJ, Inder TE 2004 Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics 114: 999–1003
Rutherford M, Counsell S, Allsop J, Boardman J, Kapellou O, Larkman D, Hajnal J, Edwards D, Cowan F 2004 Diffusion-weighted magnetic resonance imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics 114: 1004–1014
Dag Y, Firat AK, Karakas HM, Alkan A, Yakinci C, Erdem G 2006 Clinical outcomes of neonatal hypoxic ischemic encephalopathy evaluated with diffusion-weighted magnetic resonance imaging. Diagn Interv Radiol 12: 109–114
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE 2006 Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–1505
Thayyil S, Chandrasekaram M, Taylor A, Cady E, Chong K, Murad S, Omar R, Robertson NJ 2010 Cerebral magnetic resonance biomarkers for predicting long-term neurodevelopmental outcome following neonatal encephalopathy: a meta-analysis. Pediatrics 125: e382–e395
Thoresen M, Penrice J, Lorek A, Cady EB, Wylezinska M, Kirkbride V, Cooper CE, Brown GC, Edwards AD, Wyatt JS 1995 Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 37: 667–670
Blumberg RM, Cady EB, Wigglesworth JS, McKenzie JE, Edwards AD 1997 Relation between delayed impairment of cerebral energy metabolism and infarction following transient focal hypoxia-ischaemia in the developing brain. Exp Brain Res 113: 130–137
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Imperial College Healthcare Biomedical Research Centre.
Rights and permissions
About this article
Cite this article
Porter, E., Counsell, S., Edwards, A. et al. Tract-Based Spatial Statistics of Magnetic Resonance Images to Assess Disease and Treatment Effects in Perinatal Asphyxial Encephalopathy. Pediatr Res 68, 205–209 (2010). https://doi.org/10.1203/PDR.0b013e3181e9f1ba
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181e9f1ba
This article is cited by
-
Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial: Correspondence
Indian Journal of Pediatrics (2022)
-
NutriBrain: protocol for a randomised, double-blind, controlled trial to evaluate the effects of a nutritional product on brain integrity in preterm infants
BMC Pediatrics (2021)
-
Differential effects of slow rewarming after cerebral hypothermia on white matter recovery after global cerebral ischemia in near-term fetal sheep
Scientific Reports (2019)
-
Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia
Pediatric Research (2017)
-
Association between preterm brain injury and exposure to chorioamnionitis during fetal life
Scientific Reports (2016)