Abstract
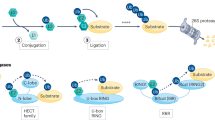
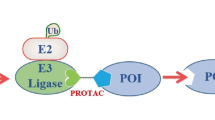
Protein degradation is the cell's mechanism of eliminating misfolded or unwanted proteins. The pathway by which proteins are degraded occurs through the ubiquitin-proteasome system. Ubiquitin is a small 9-kD (kDa) protein that is attached to proteins. A minimum of four ubiquitins are required for proteins to be recognized by the degradation machinery, known as the 26S proteasome. Defects in ubiquitination have been identified in a number of diseases, including cancer, neurodegenerative diseases, and metabolic disorders. We sought to exploit the delicate balance between protein synthesis and degradation to treat cancer by designing a chimeric molecule, known as Protac (Proteolysis Targeting Chimeric molecule). Protacs are heterobifunctional nanomolecules that are approximately 10 nm in size and can recruit proteins that cause cancer to the ubiquitin-proteasome machinery for degradation. In this review, we discuss the development of this novel technology for the treatment of cancer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AHR:
-
aryl hydrocarbon receptor
- AR:
-
androgen receptor
- ER:
-
estrogen receptor
- GFP:
-
green fluorescent protein
- HIF1:
-
hypoxia inducible factor
- MetAP-2:
-
Methionine aminopeptidase-2
- Protac:
-
Proteolysis Targeting Chimeric Molecule
- SCF:
-
Skp1-Cullin-F-box protein
- UPS:
-
ubiquitin proteasome system
- VHL:
-
Von Hippel Lindau
References
Pray TR, Parlati F, Huang J, Wong BR, Payan DG, Bennett MK, Issakani SD, Molineaux S, Demo SD 2002 Cell cycle regulatory E3 ubiquitin ligases as anticancer targets. Drug Resist Updat 5: 249–258
Vlachostergios PJ, Patrikidou A, Daliani DD, Papandreou CN 2009 The ubiquitin-proteasome system in cancer, a major player in DNA Repair. Part 2: Transcriptional regulation. J Cell Mol Med, in press
Kirkin V, McEwan DG, Novak I, Dikic I 2009 A role for ubiquitin in selective autophagy. Mol Cell 34: 259–269
Rotin D, Kumar S 2009 Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 10: 398–409
Sakamoto KM 2002 Ubiquitin-dependent proteolysis: its role in human diseases and the design of therapeutic strategies. Mol Genet Metab 77: 44–56
Reyes-Turcu FE, Ventii KH, Wilkinson KD 2009 Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78: 363–397
Mani A, Gelmann EP 2005 The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol 23: 4776–4789
Deshaies RJ, Joazeiro CA 2009 RING domain E3 ubiquitin ligases. Annu Rev Biochem 78: 399–434
Glickman MH, Ciechanover A 2002 The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428
Hershko A, Ciechanover A 1998 The ubiquitin system. Annu Rev Biochem 67: 425–479
Handley PM, Mueckler M, Siegel NR, Ciechanover A, Schwartz AL 1991 Molecular cloning, sequence, and tissue distribution of the human ubiquitin-activating enzyme E1. Proc Natl Acad Sci USA 88: 258–262
Nalepa G, Rolfe M, Harper JW 2006 Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov 5: 596–613
Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, Tamura A, Honda H, Sakata-Yanagimoto M, Kumano K, Oda H, Yamagata T, Takita J, Gotoh N, Nakazaki K, Kawamata N, Onodera M, Nobuyoshi M, Hayashi Y, Harada H, Kurokawa M, Chiba S, Mori H, Ozawa K, Omine M, Hirai H, Nakauchi H, Koeffler HP, Ogawa S 2009 Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature 460: 904–908
Zhou P, Bogacki R, McReynolds L, Howley PM 2000 Harnessing the ubiquitination machinery to target the degradation of specific cellular proteins. Mol Cell 6: 751–756
Sakamoto KM 2005 Chimeric molecules to target proteins for ubiquitination and degradation. Methods Enzymol 399: 833–847
Schneekloth JS Jr, Fonseca FN, Koldobskiy M, Mandal A, Deshaies R, Sakamoto K, Crews CM 2004 Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc 126: 3748–3754
Kaelin WG J 2007 The von hippel-lindau tumor suppressor protein: an update. Methods Enzymol 435: 371–383
Kaelin WG J 2008 The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 8: 865–873
Griffith EC, Su Z, Turk BE, Chen S, Chang YH, Wu Z, Biemann K, Liu JO 1997 Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol 4: 461–471
Li X, Chang YH 1995 Amino-terminal protein processing in Saccharomyces cerevisiae is an essential function that requires two distinct methionine aminopeptidases. Proc Natl Acad Sci USA 92: 12357–12361
Sin N, Meng L, Wang MQ, Wen JJ, Bornmann WG, Crews CM 1997 The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc Natl Acad Sci USA 94: 6099–6103
Yeh ET 2008 SUMOylation and De-SUMOylation: wrestling with life's processes. J Biol Chem 284: 8223–8227
Ben-Neriah Y 2002 Regulatory functions of ubiquitination in the immune system. Nat Immunol 3: 20–26
Chu WM, Ostertag D, Li ZW, Chang L, Chen Y, Hu Y, Williams B, Perrault J, Karin M 1999 JNK2 and IKKbeta are required for activating the innate response to viral infection. Immunity 11: 721–731
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ 2001 Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA 98: 8554–8559
Howell A 2002 Selective oestrogen receptor downregulator. Eur J Cancer 38: S61–S62
Howell A, Howell SJ, Evans DG 2003 New approaches to the endocrine prevention and treatment of breast cancer. Cancer Chemother Pharmacol 52: S39–S44
Howell SJ, Johnston SR, Howell A 2004 The use of selective estrogen receptor modulators and selective estrogen receptor down-regulators in breast cancer. Best Pract Res Clin Endocrinol Metab 18: 47–66
Debes JD, Schmidt LJ, Huang H, Tindall DJ 2002 p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer Res 62: 5632–5636
Debes JD, Tindall DJ 2002 The role of androgens and the androgen receptor in prostate cancer. Cancer Lett 187: 1–7
Sakamoto KM, Kim KB, Verma R, Ransick A, Stein B, Crews CM, Deshaies RJ 2003 Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteomics 2: 1350–1358
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ 2001 C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54
Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB 2000 The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci USA 97: 13003–13008
Kirschberg TA, VanDeusen CL, Rothbard JB, Yang M, Wender PA 2003 Arginine-based molecular transporters: the synthesis and chemical evaluation of releasable taxol-transporter conjugates. Org Lett 5: 3459–3462
Rodriguez-Gonzalez A, Cyrus K, Salcius M, Kim K, Crews CM, Deshaies RJ, Sakamoto KM 2008 Targeting steroid hormone receptors for ubiquitination and degradation in breast and prostate cancer. Oncogene 27: 7201–7211
Tang YQ, Han BM, Yao XQ, Hong Y, Wang Y, Zhao FJ, Yu SQ, Sun XW, Xia SJ 2009 Chimeric molecules facilitate the degradation of androgen receptors and repress the growth of LNCaP cells. Asian J Androl 11: 119–126
Puppala D, Lee H, Kim KB, Swanson HI 2008 Development of an aryl hydrocarbon receptor antagonist using the proteolysis-targeting chimeric molecules approach: a potential tool for chemoprevention. Mol Pharmacol 73: 1064–1071
Schneekloth AR, Pucheault M, Tae HS, Crews CM 2008 Targeted intracellular protein degradation induced by a small molecule: en route to chemical proteomics. Bioorg Med Chem Lett 18: 5904–5908
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA 2004 In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848
Heuze ML, Lamsoul I, Moog-Lutz C, Lutz PG 2008 Ubiquitin-mediated proteasomal degradation in normal and malignant hematopoiesis. Blood Cells Mol Dis 40: 200–210
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the NIH (R21 CA108545) and Department of Defense (USA) Prostate Cancer Research Program (W81XWH-06-1-0192).
Rights and permissions
About this article
Cite this article
Sakamoto, K. Protacs for Treatment of Cancer. Pediatr Res 67, 505–508 (2010). https://doi.org/10.1203/PDR.0b013e3181d35017
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/PDR.0b013e3181d35017
This article is cited by
-
Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy
Journal of Hematology & Oncology (2020)
-
Degrading proteins in animals: “PROTAC”tion goes in vivo
Cell Research (2019)
-
PROTACs: great opportunities for academia and industry
Signal Transduction and Targeted Therapy (2019)
-
The chimeric ubiquitin ligase SH2-U-box inhibits the growth of imatinib-sensitive and resistant CML by targeting the native and T315I-mutant BCR-ABL
Scientific Reports (2016)