Abstract
Background:
The effect of birth location on hypothermia-related outcomes has not been rigorously examined in the literature. In this study, we determined whether birth location had an impact on the benefits of whole-body cooling to 33.5 °C for 72 h in term infants (n = 208) with hypoxic–ischemic encephalopathy (HIE) who participated in the Neonatal Research Network (NRN) randomized controlled trial.
Methods:
Heterogeneity by birth location was examined with respect to cooling treatment for the 18-mo primary outcomes (death, moderate disability, severe disability) and secondary outcomes (death, components of disability), and in-hospital organ dysfunction. Logistic regression models were used to generate adjusted odds ratios.
Results:
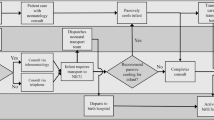
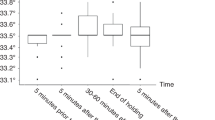
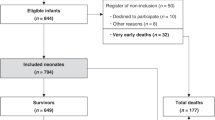
Infants born at a location other than an NRN center (outborn) (n = 93) experienced significant delays in initiation of therapy (mean (SD): 5.5 (1.1) vs. 4.4 (1.2) h), lower baseline temperatures (36.6 (1.2) vs. 37.1 (0.9) °C), and more severe HIE (43 vs. 29%) than infants born in an NRN center (inborn) (n = 115). Maternal education <12 y (50 vs. 14%) and African-American ethnicity (43 vs. 25%) were more common in the inborn group. When adjusted for NRN center and HIE severity, there were no significant differences in 18-mo outcomes or in-hospital organ dysfunction between inborn and outborn infants.
Conclusion:
Although limited by sample size and some differences in baseline characteristics, the study showed that birth location does not appear to modify the treatment effect of hypothermia after HIE.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P . Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007;4:CD003311.
Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84.
Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Jacobs SE, Morley CJ, Inder TE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med 2011;165:692–700.
Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol 2005;32:11–7.
Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol 2005;32:18–24.
Shankaran S, Pappas A, Laptook AR, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics 2008;122:e791–8.
Azzopardi D, Strohm B, Edwards AD, et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed 2009;94:F260–4.
Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010;340:c363.
Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics 2008;122:491–9.
Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics 2007;119:912–21.
Gunn AJ . Cerebral hypothermia for prevention of brain injury following perinatal asphyxia. Curr Opin Pediatr 2000;12:111–5.
Fairchild K, Sokora D, Scott J, Zanelli S . Therapeutic hypothermia on neonatal transport: 4-year experience in a single NICU. J Perinatol 2010;30:324–9.
Hallberg B, Olson L, Bartocci M, Edqvist I, Blennow M . Passive induction of hypothermia during transport of asphyxiated infants: a risk of excessive cooling. Acta Paediatr 2009;98:942–6.
Johnston ED, Becher JC, Mitchell AP, Stenson BJ . Provision of servo-controlled cooling during neonatal transport. Arch Dis Child Fetal Neonatal Ed 2011; e-pub ahead of print 6 March 2011.
Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA . Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials 2010;11:85.
Gabler NB, Duan N, Liao D, Elmore JG, Ganiats TG, Kravitz RL . Dealing with heterogeneity of treatment effects: is the literature up to the challenge? Trials 2009;10:43.
Acknowledgements
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study: NRN Steering Committee Chairs: Alan H. Jobe, University of Cincinnati; Michael S. Caplan, University of Chicago, Pritzker School of Medicine. Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island: William Oh, Betty R. Vohr, Angelita M. Hensman, and Lucy Noel. Case Western Reserve University, Rainbow Babies & Children’s Hospital: Avroy A. Fanaroff, Deanne E. Wilson-Costello, Nancy S. Newman, and Bonnie S. Siner. Cincinnati Children’s Hospital Medical Center, University Hospital and Good Samaritan Hospital: Kurt Schibler, Edward F. Donovan, Jean J. Steichen, Barbara Alexander, Cathy Grisby, Marcia Worley Mersmann, Holly L. Mincey, Jody Hessling, and Teresa L. Gratton. Duke University, School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital: C. Michael Cotten, Ricki F. Goldstein, Kathy J. Auten, and Melody B. Lohmeyer. Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown: Barbara J. Stoll, Lucky Jain, Ira Adams-Chapman, and Ellen C. Hale. Eunice Kennedy Shriver National Institute of Child Health and Human Development: Linda L. Wright and Elizabeth M. McClure. Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services: Brenda B. Poindexter, James A. Lemons, Anna M. Dusick, Lucy C. Miller, Leslie Richard. RTI International: W. Kenneth Poole, Dennis Wallace, Jane Hammond, Betty K. Hastings, Jamie E. Newman, Carolyn M. Petrie Huitema, and Jeanette O’Donnell Auman. Stanford University and Lucile Packard Children’s Hospital: Krisa P. Van Meurs, David K. Stevenson, Susan R. Hintz, William D. Rhine, M. Bethany Ball, Anne M. DeBattista, Barry E. Fleisher, Jean G. Kohn, Joan M. Baran, and Julie C. Lee-Ancajas. University of Alabama at Birmingham Health System and Children’s Hospital of Alabama: Waldemar A. Carlo, Myriam Peralta-Carcelen, Monica V. Collins, Shirley S. Cosby, and Vivien A. Phillips. University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns: Neil N. Finer, Yvonne E. Vaucher, Maynard R. Rasmussen, David Kaegi, Kathy Arnell, Clarence Demetrio, Martha G. Fuller, Chris Henderson, and Wade Rich. University of Miami Holtz Children’s Hospital: Shahnaz Duara, Charles R. Bauer, Ruth Everett-Thomas, and Amy Mur Worth. University of Rochester Medical Center Golisano Children’s Hospital: Ronnie Guillet, Dale L. Phelps, Gary J. Myers, Linda J. Reubens, Diane Hust, Julie Babish Johnson, Rosemary L. Jensen, Emily Kushner, Joan Merzbach, and Kelley Yost. University of Texas Southwestern, Medical Center at Dallas Parkland Health & Hospital System and Children’s Medical Center Dallas: Abbot R. Laptook, Pablo J. Sánchez, Walid A. Salhab, R. Sue Broyles, Roy J. Heyne, Susie Madison, Jackie F. Hickman, Gaynelle Hensley, Nancy A. Miller, Alicia Guzman, Janet S. Morgan, Cristin Dooley, Catherine Twell Boatman, Elizabeth Heyne, Linda A. Madden, and Sally S. Adams. University of Texas Health Science Center at Houston, Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District: Kathleen A. Kennedy, Esther G. Akpa, Patty A. Cluff, Claudia I. Franco, Anna E. Lis, Georgia E. McDavid, Maegan C. Simmons, Patti L. Pierce Tate, Nora I. Alaniz, Pamela J. Bradt, Magda Cedillo, Susan Dieterich, Terri Major-Kincade, Brenda H. Morris, and Laura L. Whitely. Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan: Yvette R. Johnson, Geraldine Muran, Deborah Kennedy, and Laura A. Goldston.Yale University, Yale-New Haven Children’s Hospital: Patricia Gettner, Monica Konstantino, JoAnn Poulsen, Elaine Romano, and Joanne Williams. All authors have made substantive intellectual contributions to the study conception and design, acquisition of data, or analysis and interpretation of data. They have all contributed to the drafting of the article or to the critical revision of it for important intellectual content, and have approved the final version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content. Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Abhik Das (DCC principal investigator) and Carla Bann (DCC statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natarajan, G., Pappas, A., Shankaran, S. et al. Effect of inborn vs. outborn delivery on neurodevelopmental outcomes in infants with hypoxic–ischemic encephalopathy: secondary analyses of the NICHD whole-body cooling trial. Pediatr Res 72, 414–419 (2012). https://doi.org/10.1038/pr.2012.103
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2012.103
This article is cited by
-
Evaluating the feasibility of a multicenter teleneonatology clinical effectiveness trial
Pediatric Research (2023)
-
Serious neonatal morbidities are associated with differences in DNA methylation among very preterm infants
Clinical Epigenetics (2020)
-
Prevention of excessive hypothermia in infants with hypoxic ischemic encephalopathy prior to admission to a quaternary care center: a neonatal outreach educational project
Journal of Perinatology (2019)