Abstract
Background:
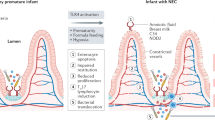
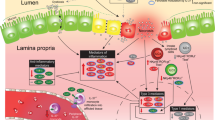
The aim of this study was to analyze whether the mucosal innate immune response of extremely-low-birth-weight (ELBW) infants might play a role in the development of necrotizing enterocolitis (NEC).
Methods:
Between April 2008 and December 2009 antimicrobial peptides were prospectively measured in fecal samples of ELBW infants. In cases requiring abdominal surgery, full-thickness gut biopsies were analyzed for expression of human β-defensin 2 (hBD2), interleukin-8 (IL-8), villin, MD2, and Toll-like receptor 4 (TLR4).
Results:
Fecal hBD1 concentrations were consistently low in all patients, whereas hBD2 concentrations were high in meconium, particularly in clinical chorioamnionitis, and then dropped, followed by a steady increase after day 14. Infants with moderate NEC showed significantly increased fecal hBD2 concentrations before clinical symptoms, in contrast to infants developing severe NEC. Analysis of intestinal resection material obtained from patients with severe NEC revealed low hBD2 mRNA and protein levels, and increased expression of the inflammatory cytokine IL-8.
Conclusion:
High hBD2 concentrations, reflecting strong intestinal immune responses, were associated with moderate courses of the disease. In severe NEC, low hBD2 expression was accompanied by low TLR4/MD2 expression, suggesting an inadequate response to luminal bacteria, possibly predisposing those infants to the development of NEC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Levy O, Martin S, Eichenwald E, et al. Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 1999;104:1327–33.
Schelonka RL, Infante AJ . Neonatal immunology. Semin Perinatol 1998;22:2–14.
Lin PW, Stoll BJ . Necrotising enterocolitis. Lancet 2006;368:1271–83.
Blakely ML, Tyson JE, Lally KP, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics 2006;117:e680–7.
Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P . Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1999;80:F167–73.
Huttner KM, Bevins CL . Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res 1999;45:785–94.
Zilbauer M, Jenke A, Wenzel G, et al. Expression of human beta-defensins in children with chronic inflammatory bowel disease. PLoS ONE 2010;5:e15389.
Schröder JM, Harder J . Human beta-defensin-2. Int J Biochem Cell Biol 1999;31:645–51.
Richter M, Topf HG, Gröschl M, et al. Influence of gestational age, cesarean section, and type of feeding on fecal human beta-defensin 2 and tumor necrosis factor-alpha. J Pediatr Gastroenterol Nutr 2010;51:103–5.
Gariboldi S, Palazzo M, Zanobbio L, et al. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J Immunol 2008;181:2103–10.
Visintin A, Mazzoni A, Spitzer JA, Segal DM . Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to Toll-like receptor 4. Proc Natl Acad Sci USA 2001;98:12156–61.
Leaphart CL, Cavallo J, Gribar SC, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 2007;179:4808–20.
Wolfs TG, Derikx JP, Hodin CM, et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis 2010;16:68–75.
Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis 2007;195:296–302.
Josefsson S, Bunn SK, Domellöf M . Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr 2007;44:407–13.
Khurana S, George SP . Regulation of cell structure and function by actin-binding proteins: villin’s perspective. FEBS Lett 2008;582:2128–39.
Kersting S, Bruewer M, Schuermann G, et al. Antigen transport and cytoskeletal characteristics of a distinct enterocyte population in inflammatory bowel diseases. Am J Pathol 2004;165:425–37.
Wang Y, Srinivasan K, Siddiqui MR, George SP, Tomar A, Khurana S . A novel role for villin in intestinal epithelial cell survival and homeostasis. J Biol Chem 2008;283:9454–64.
Niyonsaba F, Ogawa H, Nagaoka I . Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology 2004;111:273–81.
Splawski JB, Jelinek DF, Lipsky PE . Delineation of the functional capacity of human neonatal lymphocytes. J Clin Invest 1991;87:545–53.
Varoga D, Klostermeier E, Paulsen F, et al. The antimicrobial peptide HBD-2 and the Toll-like receptors-2 and -4 are induced in synovial membranes in case of septic arthritis. Virchows Arch 2009;454:685–94.
Sadeghi K, Berger A, Langgartner M, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis 2007;195:296–302.
Sodhi CP, Shi XH, Richardson WM, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 2010;138:185–96.
Tatum PM Jr, Harmon CM, Lorenz RG, Dimmitt RA . Toll-like receptor 4 is protective against neonatal murine ischemia-reperfusion intestinal injury. J Pediatr Surg 2010;45:1246–55.
Soto E, Espinoza J, Nien JK, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2007;20:15–22.
Iavazzo C, Tassis K, Gourgiotis D, et al. The role of human beta defensins 2 and 3 in the second trimester amniotic fluid in predicting preterm labor and premature rupture of membranes. Arch Gynecol Obstet 2010;281:793–9.
Campeotto F, Baldassarre M, Laforgia N, et al. Fecal expression of human β-defensin-2 following birth. Neonatology 2010;98:365–9.
McCray PB Jr, Bentley L . Human airway epithelia express a beta-defensin. Am J Respir Cell Mol Biol 1997;16:343–9.
Starner TD, Agerberth B, Gudmundsson GH, McCray PB Jr . Expression and activity of beta-defensins and LL-37 in the developing human lung. J Immunol 2005;174:1608–15.
Jia HP, Starner T, Ackermann M, Kirby P, Tack BF, McCray PB Jr . Abundant human beta-defensin-1 expression in milk and mammary gland epithelium. J Pediatr 2001;138:109–12.
Naess-Andresen CF, Egelandsdal B, Fagerhol MK . Calcium binding and concomitant changes in the structure and heat stability of calprotectin (L1 protein). Clin Mol Pathol 1995;48:M278–84.
Kliegman RM, Walsh MC . Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987;17:213–88.
Schröder O, Naumann M, Shastri Y, Povse N, Stein J . Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther 2007;26:1035–42.
Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:RESEARCH0034.
Acknowledgements
The authors thank Claudia Foerster as well as Silvia Vogel (technicians) for their excellent technical support. hBD2 antibodies were kindly donated by Tomas Ganz (UCLA). All authors contributed significantly to this work: A.C.W.J. and J.P. were involved in study design, experimental analysis, coordination, and data interpretation. M.Z. and S.W. were involved in study design, coordination, and data interpretation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jenke, A., Zilbauer, M., Postberg, J. et al. Human β-defensin 2 expression in ELBW infants with severe necrotizing enterocolitis. Pediatr Res 72, 513–520 (2012). https://doi.org/10.1038/pr.2012.110
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2012.110
This article is cited by
-
Values of serum intestinal fatty acid-binding protein, fecal calprotectin, and fecal human β-defensin 2 for predicting necrotizing enterocolitis
BMC Pediatrics (2024)
-
Human β-defensin-3 reduces excessive autophagy in intestinal epithelial cells and in experimental necrotizing enterocolitis
Scientific Reports (2019)
-
Protective effects of amniotic fluid in the setting of necrotizing enterocolitis
Pediatric Research (2017)
-
Elevated DMBT1 levels in neonatal gastrointestinal diseases
Histochemistry and Cell Biology (2016)
-
Are EGF and TLR-4 crucial to understanding the link between milk and NEC?
Mucosal Immunology (2015)