Abstract
Introduction:
Oxygen exposure plays an important role in the pathogenesis of bronchopulmonary dysplasia (BPD). The phosphodiesterase inhibitor pentoxifylline (PTX) has anti-inflammatory and antifibrotic effects in multiple organs. It was hypothesized that PTX would have a protective effect on hyperoxia-induced lung injury (HILI).
Methods:
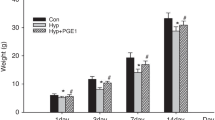
Newborn Sprague-Dawley rats were exposed to >95% oxygen (O2) and injected subcutaneously with normal saline (NS) or PTX (75 mg/kg) twice a day for 9 d. NS-injected, room air–exposed pups were controls. At days 4 and 9, lung tissue was collected to assess edema, antioxidant enzyme (AOE) activities, and vascular endothelial growth factor (VEGF) expression. At day 9, pulmonary macrophage infiltration, vascularization, and alveolarization were also examined.
Results:
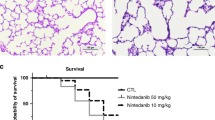
At day 9, treatment with PTX significantly increased survival from 54% to 88% during hyperoxia. Treatment with PTX significantly decreased lung edema and macrophage infiltration. PTX treatment increased lung AOE activities including those of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPX). Furthermore, PTX treatment also increased the gene expression of VEGF189 and VEGF165, increased VEGF protein expression, and improved pulmonary vascularization.
Discussion:
These data indicate that the reduced lung edema and inflammation, increased AOE activities, and improved vascularization may be responsible for the improved survival with PTX during hyperoxia. PTX may be a potential therapy in reducing some of the features of BPD in preterm newborns.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jobe AH, Bancalari E . Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9.
Bucher JR, Roberts RJ . The development of the newborn rat lung in hyperoxia: a dose-response study of lung growth, maturation, and changes in antioxidant enzyme activities. Pediatr Res 1981;15:999–1008.
Koo HC, Davis JM, Li Y, et al. Effects of transgene expression of superoxide dismutase and glutathione peroxidase on pulmonary epithelial cell growth in hyperoxia. Am J Physiol Lung Cell Mol Physiol 2005;288:L718–26.
Wilborn AM, Evers LB, Canada AT . Oxygen toxicity to the developing lung of the mouse: role of reactive oxygen species. Pediatr Res 1996;40:225–32.
Yam J, Frank L, Roberts RJ . Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. Pediatr Res 1978;12:115–9.
Frank L, Groseclose EE . Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res 1984;18:240–4.
Gerdin E, Tydén O, Eriksson UJ . The development of antioxidant enzymatic defense in the perinatal rat lung: activities of superoxide dismutase, glutathione peroxidase, and catalase. Pediatr Res 1985;19:687–91.
Frank L, Sosenko IR . Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. Pediatr Res 1991;29:292–6.
Maniscalco WM, Watkins RH, D’Angio CT, Ryan RM . Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 1997;16:557–67.
Thébaud B, Abman SH . Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007;175:978–85.
Watkins RH, D’Angio CT, Ryan RM, Patel A, Maniscalco WM . Differential expression of VEGF mRNA splice variants in newborn and adult hyperoxic lung injury. Am J Physiol 1999;276:L858–67.
Hsia CCW, Berberich MA, Driscoll B, et al. American Thoracic Society Documents. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 2004;170:319–43.
Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH . Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L1068–78.
Harris E, Schulzke SM, Patole SK . Pentoxifylline in preterm neonates: a systematic review. Paediatr Drugs 2010;12:301–11.
Michetti C, Coimbra R, Hoyt DB, Loomis W, Junger W, Wolf P . Pentoxifylline reduces acute lung injury in chronic endotoxemia. J Surg Res 2003;115:92–9.
Yada-Langoi MM, Macae M, Coimbra R, et al. Hypertonic saline and pentoxifylline prevent lung injury and bacterial translocation after hemorrhagic shock. Shock 2000;14:594–8.
Savas S, Delibas N, Savas C, Sütçü R, Cindas A . Pentoxifylline reduces biochemical markers of ischemia-reperfusion induced spinal cord injury in rabbits. Spinal Cord 2002;40:224–9.
Smalling WE Jr, Suguihara C, Huang J, Rodriguez MM, Bancalari E . Protective effect of pentoxifylline on volume-induced lung injury in newborn piglets. Biol Neonate 2004;86:15–21.
Korhonen K, Kiuru A, Svedström E, Kääpä P . Pentoxifylline reduces regional inflammatory and ventilatory disturbances in meconium-exposed piglet lungs. Pediatr Res 2004;56:901–6.
Lindsey HJ, Kisala JM, Ayala A, Lehman D, Herdon CD, Chaudry IH . Pentoxifylline attenuates oxygen-induced lung injury. J Surg Res 1994;56:543–8.
ter Horst SA, Wagenaar GT, de Boer E, et al. Pentoxifylline reduces fibrin deposition and prolongs survival in neonatal hyperoxic lung injury. J Appl Physiol 2004;97:2014–9.
Davis JM . Superoxide dismutase: a role in the prevention of chronic lung disease. Biol Neonate 1998;74:Suppl 1:29–34.
Tanswell AK, Freeman BA . Pulmonary antioxidant enzyme maturation in the fetal and neonatal rat. I. Developmental profiles. Pediatr Res 1984;18:584–7.
Ilizarov AM, Koo HC, Kazzaz JA, et al. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol 2001;24:436–41.
Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600–7.
Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM . Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–80.
Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H . Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 2002;282:L811–23.
Galambos C, Ng YS, Ali A, et al. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol 2002; 27:194–203.
Greenberg JM, Thompson FY, Brooks SK, et al. Mesenchymal expression of vascular endothelial growth factors d and A defines vascular patterning in developing lung. Dev Dyn 2002;224:144–53.
Chen CM, Wang LF, Chou HC, Lang YD, Lai YP . Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res 2007;62:128–33.
Alapati D, Rong M, Chen S, et al. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol 2011;45:1169–77.
Lin SL, Chen RH, Chen YM, et al. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol 2005;16:2702–13.
Raetsch C, Jia JD, Boigk G, et al. Pentoxifylline downregulates profibrogenic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut 2002;50:241–7.
Yang S, Zhou M, Koo DJ, Chaudry IH, Wang P . Pentoxifylline prevents the transition from the hyperdynamic to hypodynamic response during sepsis. Am J Physiol 1999;277:H1036–44.
Coimbra R, Melbostad H, Loomis W, et al. LPS-induced acute lung injury is attenuated by phosphodiesterase inhibition: effects on proinflammatory mediators, metalloproteinases, NF-kappaB, and ICAM-1 expression. J Trauma 2006;60:115–25.
Kaye AD, Ibrahim IN, Kadowitz PJ, Nossaman BD . Analysis of responses to pentoxifylline in the pulmonary vascular bed of the cat. Crit Care Med 1996;24:263–7.
McCord JM, Fridovich I . Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049–55.
Holmes RS, Masters CJ . Epigenetic interconversions of the multiple forms of mouse liver catalase. FEBS Lett 1970;11:45–8.
Paglia DE, Valentine WN . Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967;70:158–69.
Schmidt G . Determination of nucleic acids by phosphorus analysis. In: Colowick SP, Kaplan NO, eds. Methods in Enzymology, vol. 3. New York: Academic Press, 1957:671–9.
Cooney TP, Thurlbeck WM . The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 1982;37:572–9.
Chen S, Rong M, Platteau A, et al. CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2011;300:L330–40.
Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ . Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-β. Am J Physiol Heart Circ Physiol 2001;280:H909–17.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almario, B., Wu, S., Peng, J. et al. Pentoxifylline and prevention of hyperoxia-induced lung injury in neonatal rats. Pediatr Res 71, 583–589 (2012). https://doi.org/10.1038/pr.2012.14
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2012.14