Abstract
Background:
Suppressor of cytokine signaling-1 and -3 (SOCS-1 and SOCS-3) are two important negative regulators in the insulin-signaling pathway, and their overexpression may aggravate insulin resistance. Subjects with insulin resistance are often obese and have increased expressions of SOCS-1 and SOCS-3. We speculated that SOCS-1 and SOCS-3 may be involved in abnormal deposition of adipose tissues during insulin resistance.
Methods:
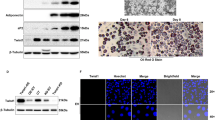
A catch-up growth intrauterine growth retardation (CG-IUGR) rat model with insulin resistance was established; mRNA and protein expression of SOCS-1, SOCS-3, the CCAAT/enhancer binding protein (C/EBPα), and peroxisome proliferator-activated receptor (PPARγ) in adipose tissue were measured by real-time PCR and western blot; plasmids carrying small hairpin RNAs (shRNAs) targeting the SOCS-1 and SOCS-3 genes were constructed and transfected into preadipocytes, which were then induced to mature. At 72 h after differentiation was induced, the expressions of C/EBPα and PPARγ, two important molecules promoting the differentiation of preadipocytes, were detected.
Results:
Expressions of SOCS-1, SOCS-3, C/EBPα, and PPARγ were markedly increased in adipose tissues of CG-IUGR rats, whereas the expressions of C/EBPα and PPARγ were significantly reduced after gene silencing of SOCS-1 or SOCS-3 in adipocytes.
Conclusion:
Overexpression of SOCS-1 and SOCS-3 may enhance the expression of C/EBPα and PPARγ, resulting in abnormal deposition of adipose tissues during insulin resistance.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barker DJ, Eriksson JG, Forsén T, Osmond C . Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31:1235–9.
Kaijser M, Bonamy AK, Akre O, et al. Perinatal risk factors for diabetes in later life. Diabetes 2009;58:523–6.
Pallotto EK, Kilbride HW . Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol 2006;49:257–69.
Liao L, Zheng R, Wang C, et al. The influence of down-regulation of suppressor of cellular signaling proteins by RNAi on glucose transport of intrauterine growth retardation rats. Pediatr Res 2011;69:497–503.
Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E . SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 2000;275:15985–91.
Ueki K, Kondo T, Kahn CR . Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 2004;24:5434–46.
Rui L, Yuan M, Frantz D, Shoelson S, White MF . SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 2002;277:42394–8.
Gueerr-Millo M . Adipose tissue and adipokines: for better or worse. Diabetes Metab 2004;30:13–9.
Gumel M . Peroxisome proliferator-activated receptor gamma and the regulation of adipocyte function: lessons from human genetic studies. Best Pract Res Clin Endocrinol Metab 2005;19:501–23.
Qiao L, Maclean PS, Schaack J, et al. C/EBPα regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 2005;54:1744–54.
Hediger ML, Overpeck MD, Maurer KR, Kuczmarski RJ, McGlynn A, Davis WW . Growth of infants and young children born small or large for gestational age: findings from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med 1998;152:1225–31.
Tamori Y, Masugi J, Nishino N, Kasuga M . Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes 2002;51:2045–55.
Ntambi JM, Young-Cheul K . Adipocyte differentiation and gene expression. J Nutr 2000;130:3122S–6S.
Peraldi P, Filloux C, Emanuelli B, Hilton DJ, Van Obberghen E . Insulin induces suppressor of cytokine signaling-3 tyrosine phosphorylation through janus-activated kinase. J Biol Chem 2001;276:24614–20.
Ahluwalia M, Evans M, Morris K, et al. The influence of the Pro12Ala mutation of the PPAR-γ receptor gene on metabolic and clinical characteristics in treatment-naïve patients with type 2 diabetes. Diabetes Obes Metab 2002;4:376–8.
Enriori PJ, Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 2007;5:181–94.
Lönnqvist F, Arner P, Nordfors L, Schalling M . Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med 1995;1:950–3.
Kondo H, Shimomura I, Matsukawa Y, et al. Association of adiponectin mutation with type 2 diabetes: a candidate gene for the insulin resistance syndrome. Diabetes 2002;51:2325–8.
Rodbell M . Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964;239:375–80.
Yu ZW, Eriksson JW . The upregulating effect of insulin and vanadate on cell surface insulin receptors in rat adipocytes is modulated by glucose and energy availability. Horm Metab Res 2000;32:310–5.
Otto TC, Lane MD . Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 2005;40:229–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, RD., Liao, LH., Ye, J. et al. Effects of SOCS 1/3 gene silencing on the expression of C/EBPα and PPARγ during differentiation and maturation of rat preadipocytes. Pediatr Res 73, 263–267 (2013). https://doi.org/10.1038/pr.2012.190
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2012.190
This article is cited by
-
LRP6 Bidirectionally Regulates Insulin Sensitivity through Insulin Receptor and S6K Signaling in Rats with CG-IUGR
Current Medical Science (2023)
-
IUGR with infantile overnutrition programs an insulin-resistant phenotype through DNA methylation of peroxisome proliferator–activated receptor-γ coactivator-1α in rats
Pediatric Research (2015)
-
Suppressors of cytokine signaling (SOCS) and type 2 diabetes
Molecular Biology Reports (2014)