Abstract
Background:
Distribution of platelet-activating factor acetylhydrolase (PAF-AH) in lipoproteins plays important roles in the onset of inflammation and atherosclerosis. We hypothesized that women with pre-eclampsia (PE), showing signs of inflammation and oxidative stress, and their fetuses have aberrations of PAF-AH activity and distribution.
Methods:
Maternal and fetal plasma PAF-AH activity, high-density lipoprotein (HDL)-associated PAF-AH (H-PAF-AH) and low-density lipoprotein (LDL)-associated PAF-AH (L-PAF-AH) were examined in women with PE (n = 127) and in women with uncomplicated pregnancies (n = 88).
Results:
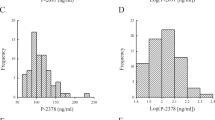
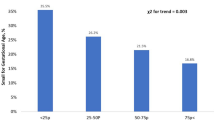
The neonates of women with severe PE (n = 42) had significantly higher plasma PAF-AH, L-PAF-AH activities, and ratio of L-PAF-AH to H-PAF-AH activities than the neonates of women with normal pregnancies (n = 83). The mothers with severe PE (n = 106) and their neonates presented a significantly higher atherogenic index (AI) and triglyceride (TG)/HDL cholesterol (C) ratio than the control mothers and their neonates. The ratio of L-PAF-AH to H-PAF-AH activities correlated positively with TG levels, TG/HDL(C) ratio, and AI and negatively with HDL(C) levels in the neonates of women with PE.
Conclusion:
The neonates of women with severe PE presented with a chronic inflammation status, increased oxidative stress, and unfavorable lipid changes, which may potentially link to related complications responsible for oxidative stress and inflammation in later life.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rodie VA, Caslake MJ, Stewart F, et al. Fetal cord plasma lipoprotein status in uncomplicated human pregnancies and in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Atherosclerosis 2004;176:181–7.
Barden A, Ritchie J, Walters B, et al. Study of plasma factors associated with neutrophil activation and lipid peroxidation in preeclampsia. Hypertension 2001;38:803–8.
Roberts JM . Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol 1998;16:5–15.
Catarino C, Rebelo I, Belo L, et al. Fetal lipoprotein changes in pre-eclampsia. Acta Obstet Gynecol Scand 2008;87:628–34.
Laskowska M, Laskowska K, Leszczynska-Gorzelak B, Oleszczuk J . Comparative analysis of the maternal and umbilical interleukin-8 levels in normal pregnancies and in pregnancies complicated by preeclampsia with intrauterine normal growth and intrauterine growth retardation. J Matern Fetal Neonatal Med 2007;20:527–32.
Karabulut AB, Kafkasli A, Burak F, Gozukara EM . Maternal and fetal plasma adenosine deaminase, xanthine oxidase and malondialdehyde levels in pre-eclampsia. Cell Biochem Funct 2005;23:279–83.
Bulgan Kilicdag E, Ay G, Celik A, Ustundag B, Ozercan I, Simsek M . Oxidant-antioxidant system changes relative to placental-umbilical pathology in patients with preeclampsia. Hypertens Pregnancy 2005;24:147–57.
Barker DJ . A new model for the origins of chronic disease. Med Health Care Philos 2001;4:31–5.
Barker DJ . In utero programming of chronic disease. Clin Sci 1998;95:115–28.
Howlader MZ, Parveen S, Tamanna S, Khan TA, Begum F . Oxidative stress and antioxidant status in neonates born to pre-eclamptic mother. J Trop Pediatr 2009;55:363–7.
Ophir E, Dourleshter G, Hirsh Y, Fait V, German L, Bornstein J . Newborns of pre-eclamptic women: a biochemical difference present in utero. Acta Obstet Gynecol Scand 2006;85:1172–8.
Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO . Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol 2003;101:529–33.
Cooper DW, Hill JA, Chesley LC, Bryans CI . Genetic control of susceptibility to eclampsia and miscarriage. Br J Obstet Gynaecol 1988;95:644–53.
Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM . Platelet-activating factor and related lipid mediators. Annu Rev Biochem 2000;69:419–45.
Karasawa K . Clinical aspects of plasma platelet-activating factor- acetylhydrolase. Biochim Biophys Acta 2006;1761:1359–72.
Rowland BL, Vermillion ST, Roudebush WE . Elevated circulating concentrations of platelet activating factor in preeclampsia. Am J Obstet Gynecol 2000;183:930–2.
Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med 2000;343:1148–55.
Caslake MJ, Packard CJ, Suckling KE, Holmes SD, Chamberlain P, Macphee CH . Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis 2000;150:413–9.
Iribarren C . Lipoprotein-associated phospholipase A2 and cardiovascular risk: state of the evidence and future directions. Arterioscler Thromb Vasc Biol 2006;26:5–6.
Chen CH . Platelet-activating factor acetylhydrolase: is it good or bad for you? Curr Opin Lipidol 2004;15:337–41.
Okamura K, Miura S, Zhang B, et al. Ratio of LDL- to HDL-associated platelet-activating factor acetylhydrolase may be a marker of inflammation in patients with paroxysmal atrial fibrillation. Circ J 2007;71:214–9.
Caslake MJ, Packard CJ . Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol 2003;14:347–52.
Kriska T, Marathe GK, Schmidt JC, McIntyre TM, Girotti AW . Phospholipase action of platelet-activating factor acetylhydrolase, but not paraoxonase-1, on long fatty acyl chain phospholipid hydroperoxides. J Biol Chem 2007;282:100–8.
Fan P, Liu HW, Wan DH, Li Y, Song Q, Bai H . Altered distribution of plasma platelet-activating factor acetylhydrolase between high-density lipoprotein and low-density lipoprotein in patients with polycystic ovary syndrome. Fertil Steril 2009;92:2054–7.
Fan P, Liu HW, Wang XS, et al. Identification of the G994T polymorphism in exon 9 of plasma platelet-activating factor acetylhydrolase gene as a risk factor for polycystic ovary syndrome. Hum Reprod 2010;25:1288–94.
Gu Y, Burlison SA, Wang Y . PAF levels and PAF-AH activities in placentas from normal and preeclamptic pregnancies. Placenta 2006;27: 744–9.
Maki N, Magness RR, Miyaura S, Gant NF, Johnston JM . Platelet-activating factor-acetylhydrolase activity in normotensive and hypertensive pregnancies. Am J Obstet Gynecol 1993;168(1 Pt 1):50–4.
Tsimihodimos V, Karabina SA, Tambaki AP, et al. Altered distribution of platelet-activating factor- acetylhydrolase activity between LDL and HDL as a function of the severity of hypercholesterolemia. J Lipid Res 2002;43:256–63.
Ohshige A, Yoshimura T, Maeda T, Ito M, Okamura H . Increased platelet-activating factor-acetylhydrolase activity in the umbilical venous plasma of growth-restricted fetuses. Obstet Gynecol 1999;93:180–3.
Maki N, Hoffman DR, Johnston JM . Platelet-activating factor acetylhydrolase activity in maternal, fetal, and newborn rabbit plasma during pregnancy and lactation. Proc Natl Acad Sci USA 1988;85:728–32.
McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol 2005;96:399–404.
Shroff R, Syrop CH, Davis W, Van Voorhis BJ, Dokras A . Risk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril 2007;88:1389–95.
Schroeder BM; American College of Obstetricians and Gynecologists. ACOG practice bulletin on diagnosing and managing preeclampsia and eclampsia. American College of Obstetricians and Gynecologists. Am Fam Physician 2002;66:330–1.
Demirel F, Bideci A, Cinaz P, et al. Serum leptin, oxidized low density lipoprotein and plasma asymmetric dimethylarginine levels and their relationship with dyslipidaemia in adolescent girls with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2007;67:129–34.
Zhang B, Fan P, Shimoji E, et al. Inhibition of cholesteryl ester transfer protein activity by JTT-705 increases apolipoprotein E-containing high-density lipoprotein and favorably affects the function and enzyme composition of high-density lipoprotein in rabbits. Arterioscler Thromb Vasc Biol 2004;24:1910–5.
Chiba H, Akizawa K, Fujisawa S, et al. A rapid and simple quantification of human apolipoprotein E-rich high-density lipoproteins in serum. Biochem Med Metab Biol 1992;47:31–7.
Acknowledgements
This work was performed at West China Second University Hospital, Sichuan University. We thank the patients with PE and control women who donated blood samples for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, P., Liu, XH., He, GL. et al. Maternal and fetal plasma platelet-activating factor acetylhydrolase activity and distribution in pre-eclampsia. Pediatr Res 72, 426–431 (2012). https://doi.org/10.1038/pr.2012.87
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2012.87
This article is cited by
-
Effect of the R92H and A379V genotypes of platelet-activating factor acetylhydrolase on its enzyme activity, oxidative stress and metabolic profile in Chinese women with polycystic ovary syndrome
Lipids in Health and Disease (2017)
-
Decreased plasma neuregulin 4 concentration is associated with increased high-sensitivity C-reactive protein in newly diagnosed type 2 diabetes mellitus patients: a cross-sectional study
Acta Diabetologica (2017)
-
Evaluation of Lp-PLA2 mass, vitronectin and PAI-1 activity levels in patients with preeclampsia
Archives of Gynecology and Obstetrics (2015)