Abstract
Background:
Cardiac dysfunction is reported to occur after severe perinatal asphyxia. We hypothesized that anesthesia of the mother with propofol during emergency cesarean section (c-section) would result in less cardiac injury (troponin T) in preterm fetuses exposed to global severe asphyxia in utero than anesthesia with isoflurane. We tested whether propofol decreases the activity of proapoptotic caspase-3 by activating the antiapoptotic AKT kinase family and the signal transducer and activator of transcription-3 (STAT-3).
Methods:
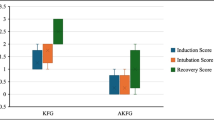
Pregnant ewes were randomized to receive either propofol or isoflurane anesthesia. A total of 44 late-preterm lambs were subjected to in utero umbilical cord occlusion (UCO), resulting in asphyxia and cardiac arrest, or sham treatment. After emergency c-section, each fetus was resuscitated, mechanically ventilated, and supported under anesthesia for 8 h using the same anesthetic as the one received by its mother.
Results:
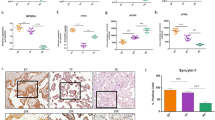
At 8 h after UCO, the fetuses whose mothers had received propofol anesthesia had lower plasma troponin T levels, and showed a trend toward a higher median left ventricular ejection fraction (LVEF) of 84% as compared with 74% for those whose mothers had received isoflurane. Postasphyxia activation of caspase-3 was lower in association with propofol anesthesia than with isoflurane. Postasphyxia levels of STAT-3 and the AKT kinase family rose 655% and 500%, respectively with the use of propofol anesthesia for the mother.
Conclusion:
The use of propofol for maternal anesthesia results in less cardiac injury in late-preterm lambs subjected to asphyxia than the use of isoflurane anesthesia. The underlying mechanism may be activation of the antiapoptotic STAT-3 and AKT pathways.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Szymankiewicz M, Matuszczak-Wleklak M, Vidyasagar D, Gadzinowski J . Retrospective diagnosis of hypoxic myocardial injury in premature newborns. J Perinat Med 2006;34:220–5.
Rennie JM, Hagmann CF, Robertson NJ . Outcome after intrapartum hypoxic ischaemia at term. Semin Fetal Neonatal Med 2007;12:398–407.
Shah P, Riphagen S, Beyene J, Perlman M . Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2004;89:F152–5.
Hankins GD, Koen S, Gei AF, Lopez SM, Van Hook JW, Anderson GD . Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol 2002;99(5 Pt 1):688–91.
Kanik E, Ozer EA, Bakiler AR, et al. Assessment of myocardial dysfunction in neonates with hypoxic-ischemic encephalopathy: is it a significant predictor of mortality? J Matern Fetal Neonatal Med 2009;22:239–42.
Pellicer A, Valverde E, Elorza MD, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 2005;115:1501–12.
Obaid L, Johnson ST, Emara M, Bigam DL, Cheung PY . Epinephrine versus dopamine to treat shock in hypoxic newborn pigs resuscitated with 100% oxygen. Shock 2008;29:262–8.
Bovill JG . Intravenous anesthesia for the patient with left ventricular dysfunction. Semin Cardiothorac Vasc Anesth 2006;10:43–8.
Vasileiou I, Xanthos T, Koudouna E, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol 2009;605:1–8.
Yildirim V, Doganci S, Aydin A, Bolcal C, Demirkilic U, Cosar A . Cardioprotective effects of sevoflurane, isoflurane, and propofol in coronary surgery patients: a randomized controlled study. Heart Surg Forum 2009;12:E1–9.
Wang B, Shravah J, Luo H, Raedschelders K, Chen DD, Ansley DM . Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun 2009;389:105–11.
Ge ZD, Pravdic D, Bienengraeber M, et al. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology 2010;112:73–85.
Kalenka A, Maurer MH, Feldmann RE, Kuschinsky W, Waschke KF . Volatile anesthetics evoke prolonged changes in the proteome of the left ventricule myocardium: defining a molecular basis of cardioprotection? Acta Anaesthesiol Scand 2006;50:414–27.
Rollins M, Lucero J . Overview of anesthetic considerations for Cesarean delivery. Br Med Bull 2012;101:105–25.
Abboud TK, Zhu J, Richardson M, Peres Da Silva E, Donovan M . Intravenous propofol vs thiamylal-isoflurane for caesarean section, comparative maternal and neonatal effects. Acta Anaesthesiol Scand 1995;39:205–9.
Ranjit MS . Cardiac abnormalities in birth asphyxia. Indian J Pediatr 2000;67:529–32.
Cruz MA, Bremmer YA, Porter BO, Gullquist SD, Watterberg KL, Rozycki HJ . Cardiac troponin T and cardiac dysfunction in extremely low-birth-weight infants. Pediatr Cardiol 2006;27:396–401.
Jensen FE . The role of glutamate receptor maturation in perinatal seizures and brain injury. Int J Dev Neurosci 2002;20:339–47.
Reed JC . Mechanisms of apoptosis. Am J Pathol 2000;157:1415–30.
Grad JM, Zeng XR, Boise LH . Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol 2000;12:543–9.
Zhuang L, Lee CS, Scolyer RA, et al. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol 2007;20:416–26.
Rees S, Harding R, Walker D . The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci 2011;29:551–63.
Eigel BN, Gursahani H, Hadley RW . ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 2004;286:H955–63.
Armstrong SC . Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res 2004;61:427–36.
Holopainen IE, Laurén HB . Glutamate signaling in the pathophysiology and therapy of prenatal insults. Pharmacol Biochem Behav 2012;100:825–34.
Eigel BN, Gursahani H, Hadley RW . Na+/Ca2+ exchanger plays a key role in inducing apoptosis after hypoxia in cultured guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 2004;287:H1466–75.
Bolli R, Stein AB, Guo Y, et al. A murine model of inducible, cardiac-specific deletion of STAT3: its use to determine the role of STAT3 in the upregulation of cardioprotective proteins by ischemic preconditioning. J Mol Cell Cardiol 2011;50:589–97.
Bray RJ . Propofol infusion syndrome in children. Paediatr Anaesth 1998;8:491–9.
Seehase M, Gantert M, Ladenburger A, et al. Myocardial response in preterm fetal sheep exposed to systemic endotoxinaemia. Pediatr Res 2011;70:242–6.
Kunzmann S, Glogger K, Been JV, et al. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol 2010;202:476.e1–9.
Acknowledgements
We thank Monique Engel for her advice and for making the technical equipment available to us. We also thank Claudia Lorefice, Elke Kuypers, Lotte van den Heuij, and the staff of the central animal facility of Maastricht University for their excellent technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seehase, M., Houthuizen, P., Jellema, R. et al. Propofol administration to the fetal–maternal unit reduces cardiac injury in late-preterm lambs subjected to severe prenatal asphyxia and cardiac arrest. Pediatr Res 73, 427–434 (2013). https://doi.org/10.1038/pr.2013.10
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2013.10
This article is cited by
-
The influence of anesthetics on substantia nigra tyrosine hydroxylase expression and tau phosphorylation in the hypoxic–ischemic near-term lamb
Pediatric Research (2018)
-
Propofol administration to the fetal–maternal unit reduces cardiac oxidative stress in preterm lambs subjected to prenatal asphyxia and cardiac arrest
Pediatric Research (2016)
-
Propofol administration to the maternal-fetal unit improved fetal EEG and influenced cerebral apoptotic pathway in preterm lambs suffering from severe asphyxia
Molecular and Cellular Pediatrics (2015)
-
Functional impairment of the auditory pathway after perinatal asphyxia and the short-term effect of perinatal propofol anesthesia in lambs
Pediatric Research (2013)