Abstract
Background:
Brown adipose tissue (BAT) thermogenesis is essential for newborn survival. Pericardial adipose tissue is a visceral depot that promotes metabolic and cardiovascular adaptations. We determined whether BAT is present in pericardial adipose tissue in newborns and whether maternal nutrition during late gestation compromises BAT in the postnatal period.
Methods:
We measured uncoupling protein 1 (UCP1) and other BAT-specific genes (e.g., β3-adrenergic receptor (β3ADR) and deiodinase type 2 (DIO2)), together with markers of white adipose tissue (WAT) in sheep on either the first or 30th day after birth. These were twin offspring born to mothers fed with either 100% or nutrient restricted (NR) to 60% of their total metabolizable requirements from 110 d gestation to term.
Results:
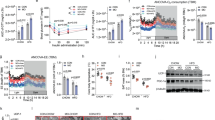
Gene expression of UCP1 and other BAT-related genes decreased significantly with age. In newborns, maternal nutrient restriction downregulated gene expression of DIO2 and the β3-adrenergic receptor with reduced UCP1 but had no effect on genes predominantly expressed in WAT.
Conclusion:
BAT is present around the heart in newborns. Exposure to a suboptimal maternal diet in late gestation specifically compromises BAT development and has the potential to place these offspring at increased risk of hypothermia after birth without effects on the subsequent appearance of WAT.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Power GG . Biology of temperature: the mammalian fetus. J Dev Physiol 1989;12:295–304.
Seale P, Lazar MA . Brown fat in humans: turning up the heat on obesity. Diabetes 2009;58:1482–4.
Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T . Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 2003;179:293–9.
Clarke L, Bryant MJ, Lomax MA, Symonds ME . Maternal manipulation of brown adipose tissue and liver development in the ovine fetus during late gestation. Br J Nutr 1997;77:871–83.
Finn D, Lomax MA, Trayhurn P . An immunohistochemical and in situ hybridisation study of the postnatal development of uncoupling protein-1 and uncoupling protein-1 mRNA in lamb perirenal adipose tissue. Cell Tissue Res 1998;294:461–6.
Budge H, Edwards LJ, McMillen IC, et al. Nutritional manipulation of fetal adipose tissue deposition and uncoupling protein 1 messenger RNA abundance in the sheep: differential effects of timing and duration. Biol Reprod 2004;71:359–65.
Budge H, Dandrea J, Mostyn A, et al. Differential effects of fetal number and maternal nutrition in late gestation on prolactin receptor abundance and adipose tissue development in the neonatal lamb. Pediatr Res 2003;53:302–8.
Mostyn A, Wilson V, Dandrea J, et al. Ontogeny and nutritional manipulation of mitochondrial protein abundance in adipose tissue and the lungs of postnatal sheep. Br J Nutr 2003;90:323–8.
Svensson PA, Jernås M, Sjöholm K, et al. Gene expression in human brown adipose tissue. Int J Mol Med 2011;27:227–32.
Festuccia WT, Blanchard PG, Deshaies Y . Control of Brown Adipose Tissue Glucose and Lipid Metabolism by PPAR? Front Endocrinol (Lausanne) 2011;2:84.
Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM . A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829–39.
Bianco AC, Silva JE . Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 1987;79:295–300.
Sacks HS, Fain JN, Holman B, et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 2009;94:3611–5.
Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012;122:1022–36.
Festuccia WT, Blanchard PG, Turcotte V, et al. The PPARgamma agonist rosiglitazone enhances rat brown adipose tissue lipogenesis from glucose without altering glucose uptake. Am J Physiol Regul Integr Comp Physiol 2009;296:R1327–35.
Anflous K, Armstrong DD, Craigen WJ . Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J Biol Chem 2001;276:1954–60.
Cannon B, Nedergaard J . Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359.
de Jesus LA, Carvalho SD, Ribeiro MO, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 2001;108:1379–85.
Klingenspor M, Ivemeyer M, Wiesinger H, Haas K, Heldmaier G, Wiesner RJ . Biogenesis of thermogenic mitochondria in brown adipose tissue of Djungarian hamsters during cold adaptation. Biochem J 1996;316 (Pt 2):607–13.
Lin J, Handschin C, Spiegelman BM . Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 2005;1:361–70.
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009;360:1500–8.
Lee P, Swarbrick MM, Zhao JT, Ho KK . Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology 2011;152:3597–602.
Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP . Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol 2001;185:93–8.
Kind KL, Roberts CT, Sohlstrom AI, et al. Chronic maternal feed restriction impairs growth but increases adiposity of the fetal guinea pig. Am J Physiol Regul Integr Comp Physiol 2005;288:R119–26.
Souza TLV, Coelho CT, Guimaraes PB, et al. Intrauterine food restriction alters the expression of uncoupling proteins in brown adipose tissue of rat newborns. J Therm Biol 2012;37:138–43.
Edwards LJ, McMillen IC . Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol (Lond) 2001;533(Pt 2):561–70.
Yuen BS, Owens PC, McFarlane JR, et al. Circulating leptin concentrations are positively related to leptin messenger RNA expression in the adipose tissue of fetal sheep in the pregnant ewe fed at or below maintenance energy requirements during late gestation. Biol Reprod 2002;67:911–6.
Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2009;90:499–504.
Gardner DS, Tingey K, Van Bon BW, et al. Programming of glucose-insulin metabolism in adult sheep after maternal undernutrition. Am J Physiol Regul Integr Comp Physiol 2005;289:R947–54.
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW . GenBank. Nucleic Acids Res 2011;39(Database issue):D32–7.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8.
Symonds ME, Bryant MJ, Clarke L, Darby CJ, Lomax MA . Effect of maternal cold exposure on brown adipose tissue and thermogenesis in the neonatal lamb. J Physiol (Lond) 1992;455:487–502.
Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150:76–85.
Acknowledgements
The authors thank Mark Pope and Victoria Wilson for their help with laboratory techniques.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ojha, S., Robinson, L., Yazdani, M. et al. Brown adipose tissue genes in pericardial adipose tissue of newborn sheep are downregulated by maternal nutrient restriction in late gestation. Pediatr Res 74, 246–251 (2013). https://doi.org/10.1038/pr.2013.107
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2013.107
This article is cited by
-
Fetal programming of adipose tissue function: an evolutionary perspective
Mammalian Genome (2014)