Abstract
Background:
Early postnatal growth retardation with subsequent catch-up growth is common in preterm infants. We describe a model of ex utero (postnatal) growth retardation followed by varying degrees of catch-up growth in the neonatal rat.
Methods:
Newborn CD rat pups were randomized to litters of 10 (NN, normal then normal intake) or 16 (R, restricted intake). On day 10, R pups were further randomized to litters of 6 (RC, restricted then catch-up intake), 10 (RN, restricted then normal intake), or 16 (RR, restricted then restricted intake). Body weight, body composition, insulin sensitivity, biochemistry, and learning (passive avoidance test) were assessed.
Results:
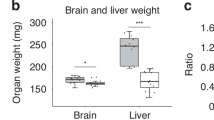
Growth was significantly lower in the R than the NN group. Subsequently, the RC group caught up with the NN group but had higher fat mass; the RN group showed partial catch-up but body composition similar to that of the NN group. Insulin sensitivity did not differ between groups. Learning behavior was significantly better in the NN than the three R groups, and in the RC group than the RR or RN groups.
Conclusion:
Early postnatal growth retardation is associated with poorer medium-term growth and poorer developmental outcome. Increased catch-up growth is associated with improved developmental outcome but with increased body adiposity, without any significant effect on glucose homeostasis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics 1999;104(2 Pt 1):280–9.
Embleton NE, Pang N, Cooke RJ . Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 2001;107:270–3.
Cooke RJ . Postdischarge nutrition of preterm infants: more questions than answers. Nestle Nutr Workshop Ser Pediatr Program 2007;59:213–24; discussion 224–8.
Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK . Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006;117:1253–61.
Hack M, Breslau N, Fanaroff AA . Differential effects of intrauterine and postnatal brain growth failure in infants of very low birth weight. Am J Dis Child 1989;143:63–8.
Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH . Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr 2003;143:163–70.
Wells JC, Chomtho S, Fewtrell M S . Programming of body composition by early growth and nutrition. Proc Nutr Soc 2007;66:423–34.
Singhal A, Fewtrell M, Cole TJ, Lucas A . Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet 2003;361:1089–97.
Zambrano E, Bautista CJ, Deás M, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol (Lond) 2006;571(Pt 1):221–30.
Zambrano E, Martínez-Samayoa PM, Bautista CJ, et al. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol (Lond) 2005;566(Pt 1):225–36.
Young JB . Effects of litter size on sympathetic activity in young adult rats. Am J Physiol Regul Integr Comp Physiol 2002;282:R1113–21.
Prior LJ, Velkoska E, Watts R, Cameron-Smith D, Morris MJ . Undernutrition during suckling in rats elevates plasma adiponectin and its receptor in skeletal muscle regardless of diet composition: a protective effect? Int J Obes (Lond) 2008;32:1585–94.
Siahanidou T, Mandyla H, Papassotiriou GP, Papassotiriou I, Chrousos G . Circulating levels of adiponectin in preterm infants. Arch Dis Child Fetal Neonatal Ed 2007;92:F286–90.
Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK . Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci 2010;32:238–48.
Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK . Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res 2006;170:224–32.
Dobbing J, Sands J . Comparative aspects of the brain growth spurt. Early Hum Dev 1979;3:79–83.
Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL . Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics 2007;5:79–94.
Heikkinen S, Argmann CA, Champy M-F, Auwerx J . Evaluation of glucose homeostasis. Curr Protocol Mol Biol 2007;2007:29B.3.1–B.3.2.
Tran TT, Chowanadisai W, Lönnerdal B, et al. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology 2002;23:645–51.
Warren LE, Horwitz BA, Hamilton JS, Fuller CA . Effects of 2 G on adiposity, leptin, lipoprotein lipase, and uncoupling protein-1 in lean and obese Zucker rats. J Appl Physiol 2001;90:606–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jou, MY., Lönnerdal, B. & Griffin, I. Effects of early postnatal growth restriction and subsequent catch-up growth on body composition, insulin sensitivity, and behavior in neonatal rats. Pediatr Res 73, 596–601 (2013). https://doi.org/10.1038/pr.2013.27
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2013.27
This article is cited by
-
Malnutrition, poor post-natal growth, intestinal dysbiosis and the developing lung
Journal of Perinatology (2021)
-
The developing gut–lung axis: postnatal growth restriction, intestinal dysbiosis, and pulmonary hypertension in a rodent model
Pediatric Research (2020)
-
Metabolic perturbations of postnatal growth restriction and hyperoxia-induced pulmonary hypertension in a bronchopulmonary dysplasia model
Metabolomics (2017)
-
Postnatal growth restriction augments oxygen-induced pulmonary hypertension in a neonatal rat model of bronchopulmonary dysplasia
Pediatric Research (2016)
-
Effects of postnatal growth restriction and subsequent catch-up growth on neurodevelopment and glucose homeostasis in rats
BMC Physiology (2015)