Abstract
Background:
Term newborns with congenital heart disease (CHD) show delayed brain development as early as the third trimester, especially in single-ventricle physiology (SVP). Mechanisms causing delayed brain development in CHD are uncertain but may include impaired fetal brain blood flow. Our objective was to determine if cardiac anatomy associated with obstruction to antegrade flow in the ascending aorta is predictive of delayed brain development as measured by diffusion tensor imaging and magnetic resonance spectroscopic (MRS) imaging.
Methods:
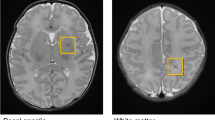
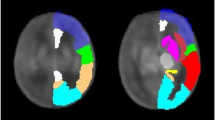
Echocardiograms from 36 term newborns with SVP were reviewed for the presence of aortic atresia and the diameter of the ascending aorta. Quantitative magnetic resonance imaging parameters measuring brain microstructural (fractional anisotropy (FA) and average diffusivity (Dav)) or metabolic development (N-acetylaspartate (NAA) and lactate/choline (Lac/Cho)) were recorded.
Results:
Increasing NAA/Cho and white matter (WM) FA, and decreasing Dav and Lac/Cho characterize normal brain development. Consistent with the hypothesis that delayed brain development is related to impaired brain perfusion, smaller ascending aortic diameter and aortic atresia were associated with higher Dav and lower WM FA. Echocardiogram variables were not associated with brain metabolic measures.
Conclusions:
These observations support the hypothesis that obstruction to fetal cerebral blood flow impairs brain microstructural development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg 2010;139:543–56.
Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002;106:12 Suppl 1:I109–14.
McQuillen PS, Hamrick SE, Perez MJ, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation 2006;113:280–5.
McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke 2007;38:Suppl 2:736–41.
Clancy RR, McGaurn SA, Goin JE, et al. Allopurinol neurocardiac protection trial in infants undergoing heart surgery using deep hypothermic circulatory arrest. Pediatrics 2001;108:61–70.
du Plessis AJ, Jonas RA, Wypij D, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg 1997;114:991–1000; discussion 1000–1.
Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg 2007;133:880–7.
Jonas RA, Wypij D, Roth SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg 2003;126:1765–74.
Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med 1993;329:1057–64.
du Plessis AJ . Neurologic complications of cardiac disease in the newborn. Clin Perinatol 1997;24:807–26.
Wernovsky G . Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young 2006;16:Suppl 1:92–104.
Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 2006;131:190–7.
Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2004;127:692–704.
Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C . Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics 1999;103:402–8.
Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010;121:26–33.
Hüppi PS, Dubois J . Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 2006;11:489–97.
Kreis R, Ernst T, Ross BD . Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med 1993;30:424–37.
Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg 2009;137:529–36; discussion 536–7.
Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007;357:1928–38.
McQuillen PS, Goff DA, Licht DJ . Effects of congenital heart disease on brain development. Prog Pediatr Cardiol 2010;29:79–85.
Richardson DK, Phibbs CS, Gray JE, McCormick MC, Workman-Daniels K, Goldmann DA . Birth weight and illness severity: independent predictors of neonatal mortality. Pediatrics 1993;91:969–75.
Beca J, Gunn J, Coleman L, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol 2009;53:1807–11.
Block AJ, McQuillen PS, Chau V, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg 2010;140:550–7.
Bartha AI, Yap KR, Miller SP, et al. The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. AJNR Am J Neuroradiol 2007;28:1015–21.
Kampmann C, Wiethoff CM, Wenzel A, et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 2000;83:667–72.
Ibuki K, Watanabe K, Yoshimura N, et al. The improvement of hypoxia correlates with neuroanatomic and developmental outcomes: comparison of midterm outcomes in infants with transposition of the great arteries or single-ventricle physiology. J Thorac Cardiovasc Surg 2012;143:1077–85.
Rosenthal GL . Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol 1996;143:505–13.
Hinton RB, Andelfinger G, Sekar P, et al. Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res 2008;64:364–9.
Shillingford AJ, Ittenbach RF, Marino BS, et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young 2007;17:189–95.
Buser JR, Maire J, Riddle A, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 2012;71:93–109.
Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol 2008;63:520–30.
Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 2005;25:5988–97.
Back SA . Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev 2006;12:129–40.
Back SA, Luo NL, Borenstein NS, Volpe JJ, Kinney HC . Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol 2002;61:197–211.
Mäkikallio K, McElhinney DB, Levine JC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation 2006;113:1401–5.
Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D . Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 1988;166(1 Pt 1):173–80.
Brody BA, Kinney HC, Kloman AS, Gilles FH . Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol 1987;46:283–301.
Kinney HC, Brody BA, Kloman AS, Gilles FH . Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol 1988;47:217–34.
Lang RM, Bierig M, Devereux RB, et al.; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63.
Gautier M, Detaint D, Fermanian C, et al. Nomograms for aortic root diameters in children using two-dimensional echocardiography. Am J Cardiol 2010;105:888–94.
Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol 2009;66:155–64.
Glenn OA . Normal development of the fetal brain by MRI. Semin Perinatol 2009;33:208–19.
Bonifacio SL, Glass HC, Chau V, et al. Extreme premature birth is not associated with impaired development of brain microstructure. J Pediatr 2010;157:726–32.e1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sethi, V., Tabbutt, S., Dimitropoulos, A. et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res 73, 661–667 (2013). https://doi.org/10.1038/pr.2013.29
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2013.29
This article is cited by
-
In infants with congenital heart disease autonomic dysfunction is associated with pre-operative brain injury
Pediatric Research (2022)
-
Aortic valve surgery: management and outcomes in the paediatric population
European Journal of Pediatrics (2021)
-
Antenatal and Perioperative Mechanisms of Global Neurological Injury in Congenital Heart Disease
Pediatric Cardiology (2021)
-
Altered brain diffusion tensor imaging indices in adolescents with the Fontan palliation
Neuroradiology (2019)
-
Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before Fontan completion
Pediatric Research (2018)