Abstract
Background:
Higher cortical function during sensory processing can be examined by recording specific somatosensory-evoked magnetic fields (SEFs) with magnetoencephalography (MEG). We evaluated whether, in extremely low-gestational-age (ELGA) infants, abnormalities in MEG-recorded SEFs at term age are associated with adverse neurodevelopment at 2 y of corrected age.
Methods:
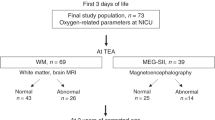
SEFs to tactile stimulation of the index finger were recorded at term age in 30 ELGA infants (26.5 ± 1.2 wk, birth weight: 884g ± 181 g). Neurodevelopment was evaluated at 2 y of corrected age. Controls were 11 healthy term infants.
Results:
In nine of the ELGA infants (30.0%), SEFs were categorized as abnormal on the basis of lack of response from secondary somatosensory cortex (SII). At 2 y, these infants had a significantly worse mean developmental quotient and locomotor subscale on the Griffiths Mental Development Scales than the ELGA infants with normal responses. Mild white matter abnormalities in magnetic resonance imaging at term age were detected in 21% of infants, but these abnormalities were not associated with adverse neurodevelopment.
Conclusion:
Abnormal SII responses at term predict adverse neuromotor development at 2 y of corrected age. This adverse development may not be foreseen with conventional neuroimaging methods, suggesting a role for evaluating SII responses in the developmental risk assessment of ELGA infants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Whittingham K, Wee D, Boyd R . Systematic review of the efficacy of parenting interventions for children with cerebral palsy. Child Care Health Dev 2011;37:475–83.
Mikkola K, Ritari N, Tommiska V, et al. Neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1996-1997. Pediatrics 2005;116:1391–400.
Salt A, Redshaw M . Neurodevelopmental follow-up after preterm birth: follow up after two years. Early Hum Dev 2006;82:185–97.
Saigal S, Doyle LW . An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9.
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE . Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–94.
Volpe JJ . Neurology of the Newborn, 5th edn. Philadelphia, PA: Saunders, 2008:172–74.
Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005;147:609–16.
Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 2006;118:536–48.
Brown NC, Inder TE, Bear MJ, Hunt RW, Anderson PJ, Doyle LW . Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr 2009;155:32–8, 38.e1.
Skiöld B, Vollmer B, Böhm B, et al. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J Pediatr 2012;160:559–566.e1.
Mirmiran M, Barnes PD, Keller K, et al. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics 2004;114:992–8.
Klimach VJ, Cooke RW . Short-latency cortical somatosensory evoked responses of preterm infants with ultrasound abnormality of the brain. Dev Med Child Neurol 1988;30:215–21.
Willis J, Duncan MC, Bell R, Pappas F, Moniz M . Somatosensory evoked potentials predict neuromotor outcome after periventricular hemorrhage. Dev Med Child Neurol 1989;31:435–9.
de Vries LS, Eken P, Pierrat V, Daniels H, Casaer P . Prediction of neurodevelopmental outcome in the preterm infant: short latency cortical somatosensory evoked potentials compared with cranial ultrasound. Arch Dis Child 1992;67(10 Spec No):1177–81.
Pierrat V, Eken P, de Vries LS . The predictive value of cranial ultrasound and of somatosensory evoked potentials after nerve stimulation for adverse neurological outcome in preterm infants. Dev Med Child Neurol 1997;39:398–403.
Volpe JJ . Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–24.
Nevalainen P, Lauronen L, Sambeth A, Wikström H, Okada Y, Pihko E . Somatosensory evoked magnetic fields from the primary and secondary somatosensory cortices in healthy newborns. Neuroimage 2008;40:738–45.
Nevalainen P, Pihko E, Metsäranta M, Andersson S, Autti T, Lauronen L . Does very premature birth affect the functioning of the somatosensory cortex?–a magnetoencephalography study. Int J Psychophysiol 2008;68:85–93.
Pihko E, Lauronen L, Kivistö K, Nevalainen P . Increasing the efficiency of neonatal MEG measurements by alternating auditory and tactile stimulation. Clin Neurophysiol 2011;122:808–14.
Nevalainen P, Pihko E, Metsäranta M, et al. Evoked magnetic fields from primary and secondary somatosensory cortices: a reliable tool for assessment of cortical processing in the neonatal period. Clin Neurophysiol 2012;123:2377–83.
Pike AA, Marlow N . The role of cortical evoked responses in predicting neuromotor outcome in very preterm infants. Early Hum Dev 2000;57:123–35.
Vanhatalo S, Lauronen L . Neonatal SEP - back to bedside with basic science. Semin Fetal Neonatal Med 2006;11:464–70.
Karniski W . The late somatosensory evoked potential in premature and term infants. I. Principal component topography. Electroencephalogr Clin Neurophysiol 1992;84:32–43.
Karniski W, Wyble L, Lease L, Blair RC . The late somatosensory evoked potential in premature and term infants. II. Topography and latency development. Electroencephalogr Clin Neurophysiol 1992;84:44–54.
Whitsel BL, Petrucelli LM, Werner G . Symmetry and connectivity in the map of the body surface in somatosensory area II of primates. J Neurophysiol 1969;32:170–83.
Simões C, Hari R . Relationship between responses to contra- and ipsilateral stimuli in the human second somatosensory cortex SII. Neuroimage 1999;10:408–16.
Huttunen J, Wikström H, Korvenoja A, Seppäläinen AM, Aronen H, Ilmoniemi RJ . Significance of the second somatosensory cortex in sensorimotor integration: enhancement of sensory responses during finger movements. Neuroreport 1996;7:1009–12.
Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE . Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol 2011;36:22–41.
Brandt I, Sticker EJ . Griffiths Entwicklungsskalen zur Beurteilung der Entwicklung in den ersten beiden Lebensjahren. Beltz Test GmbH, Göttingen, 2001.
Hempel MS . The Neurological Examination for Toddler Age. Thesis,University of Groningen: Groningen, The Netherlands, 1993.
Bayley N . The Bayley Scales of Infant and Toddler Development, Third Edition. San Antonio, TX: The Psychological Corporation, 2005.
Pihkala J, Hakala T, Voutilainen P, Raivio K . [Characteristic of recent fetal growth curves in Finland]. Duodecim 1989;105:1540–6.
Pihko E, Lauronen L, Wikström H, et al. Somatosensory evoked potentials and magnetic fields elicited by tactile stimulation of the hand during active and quiet sleep in newborns. Clin Neurophysiol 2004;115:448–55.
Taulu S, Simola J . Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 2006;51:1759–68.
Medvedovsky M, Taulu S, Bikmullina R, Ahonen A, Paetau R . Fine tuning the correlation limit of spatio-temporal signal space separation for magnetoencephalography. J Neurosci Methods 2009;177:203–11.
Hadders-Algra M . Developmental coordination disorder: is clumsy motor behavior caused by a lesion of the brain at early age? Neural Plast 2003;10:39–50.
Acknowledgements
We are deeply indebted to Marita Suni for her excellence in caring for the very prematurely born infants during the MEG measurements. Furthermore, we express our gratitude to all our subjects and their families for preparing the way for this study. Finally, we thank the personnel of the maternity ward of the Department of Obstetrics and Gynecology and the Neonatal Intensive Care Unit at the Helsinki University Central Hospital, newborns’ ward at Helsinki Maternity Hospital, and children’s ward at Jorvi Hospital for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahkonen, P., Nevalainen, P., Lauronen, L. et al. Cortical somatosensory processing measured by magnetoencephalography predicts neurodevelopment in extremely low-gestational-age infants. Pediatr Res 73, 763–771 (2013). https://doi.org/10.1038/pr.2013.46
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2013.46
This article is cited by
-
Early oxygen levels contribute to brain injury in extremely preterm infants
Pediatric Research (2021)