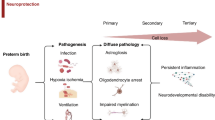
Abstract
The majority of infants born very preterm (24–32 wk gestational age) now survive; however, long-term neurodevelopmental and behavioral problems remain a concern. As part of their neonatal care, very preterm infants undergo repeated painful procedures during a period of rapid brain development and programming of stress systems. Infants born this early have the nociceptive circuitry required to perceive pain, however, their sensory systems are functionally immature. An imbalance of excitatory vs. inhibitory processes leads to increased nociceptive signaling in the central nervous system. Specific cell populations in the central nervous system of preterm neonates are particularly vulnerable to excitoxicity, oxidative stress, and inflammation. Neonatal rat models have demonstrated that persistent or repeated pain increases apoptosis of neurons, and neonatal pain and stress lead to anxiety-like behaviors during adulthood. In humans, greater exposure to neonatal pain-related stress has been associated with altered brain microstructure and stress hormone levels, as well as with poorer cognitive, motor, and behavioral neurodevelopment in infants and children born very preterm. Therefore, it is important that pain-related stress in preterm neonates is accurately identified, appropriately managed, and that pain management strategies are evaluated for protective or adverse effects in the long term.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fitzgerald M . The development of nociceptive circuits. Nat Rev Neurosci 2005;6:507–20.
Fitzgerald M, Walker SM . Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol 2009;5:35–50.
Andrews K, Fitzgerald M . The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain 1994;56:95–101.
Jennings E, Fitzgerald M . Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: a fibre-induced sensitization. J Physiol (Lond) 1998;509 (Pt 3):859–68.
Li J, Walker SM, Fitzgerald M, Baccei ML . Activity-dependent modulation of glutamatergic signaling in the developing rat dorsal horn by early tissue injury. J Neurophysiol 2009;102:2208–19.
Beggs S, Torsney C, Drew LJ, Fitzgerald M . The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci 2002;16:1249–58.
Granmo M, Petersson P, Schouenborg J . Action-based body maps in the spinal cord emerge from a transitory floating organization. J Neurosci 2008;28:5494–503.
Fitzgerald M, Millard C, McIntosh N . Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anaesthesia. Pain 1989;39:31–6.
Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK . Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks’ postconceptional age. Pediatrics 2001;107:105–12.
Holsti L, Grunau RE, Oberlander TF, Whitfield MF . Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Hum Dev 2005;81:293–302.
Holsti L, Grunau RE, Whifield MF, Oberlander TF, Lindh V . Behavioral responses to pain are heightened after clustered care in preterm infants born between 30 and 32 weeks gestational age. Clin J Pain 2006;22:757–64.
Walker SM, Tochiki KK, Fitzgerald M . Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain 2009;147:99–106.
Fabrizi L, Slater R, Worley A, et al. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Curr Biol 2011;21:1552–8.
McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex 2002;12:1237–43.
Vinall J, Grunau RE, Brant R, et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med 2013;5:168ra8.
van Praag H, Frenk H . The development of stimulation-produced analgesia (SPA) in the rat. Brain Res Dev Brain Res 1991;64:71–6.
Hathway GJ, Koch S, Low L, Fitzgerald M . The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol (Lond) 2009;587(Pt 12):2927–35.
Holsti L, Grunau RE, Oberlander TF, Osiovich H . Is it painful or not? Discriminant validity of the Behavioral Indicators of Infant Pain (BIIP) scale. Clin J Pain 2008;24:83–8.
Stevens BJ, Johnston CC, Horton L . Multidimensional pain assessment in premature neonates: a pilot study. J Obstet Gynecol Neonatal Nurs 1993;22:531–41.
Ranger M, Johnston CC, Anand KJ . Current controversies regarding pain assessment in neonates. Semin Perinatol 2007;31:283–8.
van Dijk M, Tibboel D . Update on pain assessment in sick neonates and infants. Pediatr Clin North Am 2012;59:1167–81.
Slater R, Worley A, Fabrizi L, et al. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain 2010;14:321–6.
Slater R, Cornelissen L, Fabrizi L, et al. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet 2010;376:1225–32.
Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ . Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics 2005;115:1494–500.
Johnston C, Barrington KJ, Taddio A, Carbajal R, Filion F . Pain in Canadian NICUs: have we improved over the past 12 years? Clin J Pain 2011;27:225–32.
Chrousos GP . Stress and disorders of the stress system. Nat Rev Endocrinol 2009;5:374–81.
Coplan JD, Andrews MW, Rosenblum LA, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA 1996;93:1619–23.
Lupien SJ, McEwen BS, Gunnar MR, Heim C . Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009;10:434–45.
Heim C, Nemeroff CB . Neurobiology of early life stress: clinical studies. Semin Clin Neuropsychiatry 2002;7:147–59.
Meaney MJ, Diorio J, Francis D, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci 1996;18:49–72.
Peters KL . Neonatal stress reactivity and cortisol. J Perinat Neonatal Nurs 1998;11:45–59.
Grunau RE, Holsti L, Haley DW, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain 2005;113:293–300.
Grunau RE, Weinberg J, Whitfield MF . Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics 2004;114:e77–84.
Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P . Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr 2007;150:151–6.
McEwen BS . Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 2004;1032:1–7.
Volpe JJ . Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–24.
Kostovic I, Judas M, Rados M, Hrabac P . Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 2002;12:536–44.
Kostovic I, Judas M . Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec 2002;267:1–6.
McQuillen PS, Ferriero DM . Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol 2005;15:250–60.
Ghosh A, Antonini A, McConnell SK, Shatz CJ . Requirement for subplate neurons in the formation of thalamocortical connections. Nature 1990;347:179–81.
Ghosh A, Shatz CJ . Involvement of subplate neurons in the formation of ocular dominance columns. Science 1992;255:1441–3.
McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM . Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci 2003;23:3308–15.
Qu Y, Vadivelu S, Choi L, et al. Neurons derived from embryonic stem (ES) cells resemble normal neurons in their vulnerability to excitotoxic death. Exp Neurol 2003;184:326–36.
McDonald JW, Johnston MV . Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev 1990;15:41–70.
Talos DM, Follett PL, Folkerth RD, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol 2006;497:61–77.
Deng W, Rosenberg PA, Volpe JJ, Jensen FE . Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA 2003;100:6801–6.
Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ . Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci 1998;18:6241–53.
Back SA, Luo NL, Mallinson RA, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol 2005;58:108–20.
Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol 2003;62:441–50.
Buntinx M, Moreels M, Vandenabeele F, et al. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res 2004;76:834–45.
Pang Y, Cai Z, Rhodes PG . Effect of tumor necrosis factor-alpha on developing optic nerve oligodendrocytes in culture. J Neurosci Res 2005;80:226–34.
Slater L, Asmerom Y, Boskovic DS, et al. Procedural pain and oxidative stress in premature neonates. J Pain 2012;13:590–7.
Hansson E . Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol (Oxf) 2006;187:321–7.
Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol 2012;71:385–96.
Smith GC, Gutovich J, Smyser C, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol 2011;70:541–9.
Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW . Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res 2007;62:283–90.
Dührsen L, Simons SH, Dzietko M, et al. Effects of repetitive exposure to pain and morphine treatment on the neonatal rat brain. Neonatology 2013;103:35–43.
Zwicker JG, Grunau RE, Adams E, et al. Score for neonatal acute physiology-II and neonatal pain predict corticospinal tract development in premature newborns. Pediatr Neurol 2013;48:123–129.e1.
Ranger M, Chau CM, Garg A, et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS ONE 2013;8:e76702.
Doesburg SM, Chau CM, Cheung TP, et al. Neonatal pain-related stress, functional cortical activity and visual-perceptual abilities in school-age children born at extremely low gestational age. Pain 2013;154:1946–52.
Grunau RE, Whitfield MF, Petrie-Thomas J, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 2009;143:138–46.
Vinall J, Miller SP, Synnes AR, Grunau RE . Parent behaviors moderate the relationship between neonatal pain and internalizing behaviors at 18 months corrected age in children born very prematurely. Pain 2013;154:1831–9.
Ranger M, Synnes AR, Vinall J, Grunau RE . Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur J Pain 2013 (doi:10.1002/j.1532-2149.2013.00431.x).
Anand KJ . Pharmacological approaches to the management of pain in the neonatal intensive care unit. J Perinatol 2007;27:Suppl 1:S4–S11.
Durrmeyer X, Vutskits L, Anand KJ, Rimensberger PC . Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr Res 2010;67:117–27.
McPherson CC, Grunau RE . Neonatal pain control and neurologic effects of anesthetics and sedatives in preterm infants. Clin Perinatol 2014;41:209–27.
Marlow N . Anesthesia and long-term outcomes after neonatal intensive care unit. Paediatr Anaesth 2014;24:60–7.
Bellù R, de Waal KA, Zanini R . Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev 2008;1:CD004212.
Batton DG, Barrington KJ, Wallman C . Prevention and management of pain in the neonate: an update. Pediatrics 2006;118:2231–41.
Pillai Riddell RR, Racine NM, Turcotte K, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev 2011;5:CD006275.
Stevens B, Yamada J, Lee GY, Ohlsson A . Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2013;1:CD001069.
Taddio A, Yiu A, Smith RW, Katz J, McNair C, Shah V . Variability in clinical practice guidelines for sweetening agents in newborn infants undergoing painful procedures. Clin J Pain 2009;25:153–5.
Milgrom J, Newnham C, Anderson PJ, et al. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res 2010;67:330–5.
Brummelte S, Grunau RE, Zaidman-Zait A, Weinberg J, Nordstokke D, Cepeda IL . Cortisol levels in relation to maternal interaction and child internalizing behavior in preterm and full-term children at 18 months corrected age. Dev Psychobiol 2011;53:184–95.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vinall, J., Grunau, R. Impact of repeated procedural pain-related stress in infants born very preterm. Pediatr Res 75, 584–587 (2014). https://doi.org/10.1038/pr.2014.16
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2014.16
This article is cited by
-
Efficacy and safety of dexmedetomidine for analgesia and sedation in neonates: a systematic review
Journal of Perinatology (2024)
-
Effect of Splint Application on the Functional Duration of Peripheral Intravenous Cannulation in Neonates: A Systematic Review and Meta-analysis
Indian Pediatrics (2024)
-
Experience of early-life pain in premature infants is associated with atypical cerebellar development and later neurodevelopmental deficits
BMC Medicine (2023)
-
COMFORTneo scale: a reliable and valid instrument to measure prolonged pain in neonates?
Journal of Perinatology (2023)
-
Lärmbelastung einer neonatologischen Intensivstation
Zentralblatt für Arbeitsmedizin, Arbeitsschutz und Ergonomie (2023)