Abstract
Background:
A proposed etiology of biliary atresia (BA) entails a virus-induced, progressive immune-mediated injury of the biliary system. Intravenous Ig (IVIg) has demonstrated clinical benefit in several inflammatory diseases. The aim of this study was to determine the therapeutic effects of high-dose IgG treatment in the rhesus rotavirus (RRV)–induced mouse model of BA.
Methods:
Newborn mice were infected with RRV, and jaundiced mice were given high-dose IgG or albumin control. Survival, histology, direct bilirubin, liver immune cell subsets, and cytokine production were analyzed.
Results:
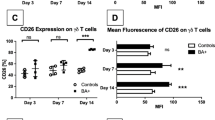
There was no difference in overall survival between RRV-infected groups, however high-dose IgG resulted in decreased bilirubin, bile duct inflammation, and increased extrahepatic bile duct patency. High-dose IgG decreased vascular cell adhesion molecule-1, resulting in limited migration of immune cells to portal tracts. High-dose IgG significantly decreased CD4+ T cell production of interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α and CD8+ T cell production of IFN-γ, as well as increased levels of regulatory T cells.
Conclusion:
High-dose IgG therapy in murine BA dramatically decreased Th1 cell-mediated inflammation and biliary obstruction. This study lends support for consideration of IVIg clinical trials in infants with BA, to diminish the progressive intrahepatic bile duct injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Davenport M . Biliary atresia: clinical aspects. Semin Pediatr Surg 2012;21:175–84.
Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI . International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr 2013;56:344–54.
Gallo A, Esquivel CO . Current options for management of biliary atresia. Pediatr Transplant 2013;17:95–8.
Petersen C, Davenport M . Aetiology of biliary atresia: what is actually known? Orphanet J Rare Dis 2013;8:128.
Bessho K, Bezerra JA . Biliary atresia: will blocking inflammation tame the disease? Annu Rev Med 2011;62:171–85.
Mack CL, Feldman AG, Sokol RJ . Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis 2012;32:307–16.
Riepenhoff-Talty M, Schaekel K, Clark HF, et al. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr Res 1993;33(4 Pt 1):394–9.
Mack CL, Tucker RM, Sokol RJ, et al. Biliary atresia is associated with CD4+ Th1 cell-mediated portal tract inflammation. Pediatr Res 2004;56:79–87.
Mack CL, Tucker RM, Sokol RJ, Kotzin BL . Armed CD4+ Th1 effector cells and activated macrophages participate in bile duct injury in murine biliary atresia. Clin Immunol 2005;115:200–9.
Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-γ in experimental biliary atresia. J Clin Invest 2004;114:322–9.
Mack CL, Tucker RM, Lu BR, et al. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology 2006;44:1231–9.
Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L . Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmun Rev 2007;6:176–82.
Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL . a-enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology 2010;139:1753–61.
Lages CS, Simmons J, Chougnet CA, Miethke AG . Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology 2012;56:219–27.
Brindley SM, Lanham AM, Karrer FM, Tucker RM, Fontenot AP, Mack CL . Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology 2012;55:1130–8.
Tucker RM, Feldman AG, Fenner EK, Mack CL . Regulatory T cells inhibit Th1 cell-mediated bile duct injury in murine biliary atresia. J Hepatol 2013;59:790–6.
Kazatchkine MD, Kaveri SV . Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 2001;345:747–55.
Rauova L, Rovensky J, Shoenfeld Y . Immunomodulation of autoimmune diseases by high-dose intravenous immunoglobulins. Springer Semin Immunopathol 2001;23:447–57.
Gelfand EW . Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med 2012;367:2015–25.
Samuelsson A, Towers TL, Ravetch JV . Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science 2001;291:484–6.
Zhu KY, Feferman T, Maiti PK, Souroujon MC, Fuchs S . Intravenous immunoglobulin suppresses experimental myasthenia gravis: immunological mechanisms. J Neuroimmunol 2006;176:187–97.
Kishimoto C, Takada H, Kawamata H, Umatake M, Ochiai H . Immunoglobulin treatment prevents congestive heart failure in murine encephalomyocarditis viral myocarditis associated with reduction of inflammatory cytokines. J Pharmacol Exp Ther 2001;299:645–51.
Achiron A, Mor F, Margalit R, Cohen IR, Lider O, Miron S . Suppression of experimental autoimmune encephalomyelitis by intravenously administered polyclonal immunoglobulins. J Autoimmun 2000;15:323–30.
Maddur MS, Othy S, Hegde P, et al. Immunomodulation by intravenous immunoglobulin: role of regulatory T cells. J Clin Immunol 2010;30:Suppl 1:S4–8.
Gearing AJ, Newman W . Circulating adhesion molecules in disease. Immunol Today 1993;14:506–12.
Achiron A, Margalit R, Hershkoviz R, et al. Intravenous immunoglobulin treatment of experimental T cell-mediated autoimmune disease. Upregulation of T cell proliferation and downregulation of tumor necrosis factor alpha secretion. J Clin Invest 1994;93:600–5.
Andersson J, Skansén-Saphir U, Sparrelid E, Andersson U . Intravenous immune globulin affects cytokine production in T lymphocytes and monocytes/macrophages. Clin Exp Immunol 1996;104:Suppl 1:10–20.
Ober RJ, Radu CG, Ghetie V, Ward ES . Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol 2001;13:1551–9.
Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I . Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem 2010;285:4826–36.
Elangbam CS, Qualls CW Jr, Dahlgren RR . Cell adhesion molecules–update. Vet Pathol 1997;34:61–73.
Volpes R, Van Den Oord JJ, Desmet VJ . Vascular adhesion molecules in acute and chronic liver inflammation. Hepatology 1992;15:269–75.
Dillon P, Belchis D, Tracy T, Cilley R, Hafer L, Krummel T . Increased expression of intracellular adhesion molecules in biliary atresia. The Amer J Pathol 1994;145:263–7.
Kobayashi H, Horikoshi K, Long L, Yamataka A, Lane GJ, Miyano T . Serum concentration of adhesion molecules in postoperative biliary atresia patients: relationship to disease activity and cirrhosis. J Pediatr Surg 2001;36:1297–301.
Patel SJ, Jindal R, King KR, Tilles AW, Yarmush ML . The inflammatory response to double stranded DNA in endothelial cells is mediated by NF?B and TNFa. PLoS ONE 2011;6:e19910.
Skurkovich S, Skurkovich B . Anticytokine therapy, especially anti-interferon-gamma, as a pathogenetic treatment in TH-1 autoimmune diseases. Ann N Y Acad Sci 2005;1051:684–700.
Mouzaki A, Theodoropoulou M, Gianakopoulos I, Vlaha V, Kyrtsonis MC, Maniatis A . Expression patterns of Th1 and Th2 cytokine genes in childhood idiopathic thrombocytopenic purpura (ITP) at presentation and their modulation by intravenous immunoglobulin G (IVIg) treatment: their role in prognosis. Blood 2002;100:1774–9.
Svenson M, Hansen MB, Bendtzen K . Binding of cytokines to pharmaceutically prepared human immunoglobulin. J Clin Investigat 1993:92:2533–39.
Ross C, Svenson M, Nielsen H, Lundsgaard C, Hansen MB, Bendtzen K . Increased in vivo antibody activity against interferon alpha, interleukin-1alpha, and interleukin-6 after high-dose Ig therapy. Blood 1997;90:2376–80.
Ross C, Svenson M, Hansen MB, Vejlsgaard GL, Bendtzen K . High avidity IFN-neutralizing antibodies in pharmaceutically prepared human IgG. J Clin Invest 1995;95:1974–8.
Ephrem A, Chamat S, Miquel C, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood 2008;111:715–22.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
(TIFF 24653 kb)
Supplementary Figure S2
(TIFF 24653 kb)
Supplementary Figure S3
(TIFF 24653 kb)
Rights and permissions
About this article
Cite this article
Fenner, E., Boguniewicz, J., Tucker, R. et al. High-dose IgG therapy mitigates bile duct–targeted inflammation and obstruction in a mouse model of biliary atresia. Pediatr Res 76, 72–80 (2014). https://doi.org/10.1038/pr.2014.46
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2014.46
This article is cited by
-
Biliatresone induces cholangiopathy in C57BL/6J neonates
Scientific Reports (2023)
-
Intravenous Immunoglobulin (IVIg) Induce a Protective Phenotype in Microglia Preventing Neuronal Cell Death in Ischaemic Stroke
NeuroMolecular Medicine (2020)
-
Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders
Hepatology International (2020)
-
Oligoclonal immunoglobulin repertoire in biliary remnants of biliary atresia
Scientific Reports (2019)
-
Intravenous immunoglobulin for the treatment of intractable cholangitis after Kasai portoenterostomy in biliary atresia patients
Pediatric Surgery International (2018)