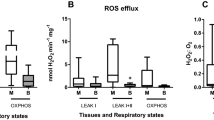
Abstract
Background:
Hypoxic–ischemic insults to the neonatal brain may cause neurodevelopmental disorders. Vulnerability of different areas of the neural tissue to hypoxic–ischemic stress might be explained by either heterogeneous sensitivity to oxygen or neuroprotective capability. Our understanding of regional heterogeneity is still incomplete in terms of metabolic reconfiguration and/or activation of neuroprotective mechanisms.
Methods:
We studied, by western blotting, reverse-transcriptase PCR, and tandem mass spectrometry, the response of retina and choroid at protein, gene, and metabolic levels during hypoxia in a piglet model of acute postnatal hypoxia.
Results:
We evidenced a metabolic shift towards glycolysis in choroid after hypoxia while retina experienced a dramatic energy stress with decreased mitochondrial metabolites. Hypoxia-inducible transcription factor-1α (HIF-1α) was not stabilized in retina during hypoxia, supported by a deficient signaling from v-akt murine thymoma viral oncogene (AKT) and ERK1/2, and unchanged glutathione redox status. In retina, but not in choroid, phosphorylation of p65 (NF-κB) and increased transcription of target genes may have a major role during hypoxic stress.
Conclusion:
We showed that the retina engages a distinct pattern of signaling and transcriptional events than observed in the choroid. Retina and choroid may reflect regional sensitivity to hypoxia. While prolonged and intense hypoxia may jeopardize retinal cell survival, choroid sets up a different pattern of response, which promotes adaptation to these adverse conditions.
Similar content being viewed by others

Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vento M, Saugstad OD . Resuscitation of the term and preterm infant. Semin Fetal Neonatal Med 2010;15:216–22.
Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010;125:e1340–7.
Delaey C, Van De Voorde J . Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res 2000;32:249–56.
Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E . Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 2008;27:284–330.
Thiersch M, Raffelsberger W, Frigg R, et al. Analysis of the retinal gene expression profile after hypoxic preconditioning identifies candidate genes for neuroprotection. BMC Genomics 2008;9:73.
Shahidi M, Wanek J, Blair NP, Mori M . Three-dimensional mapping of chorioretinal vascular oxygen tension in the rat. Invest Ophthalmol Vis Sci 2009;50:820–5.
Wanek J, Teng PY, Blair NP, Shahidi M . Inner retinal oxygen delivery and metabolism under normoxia and hypoxia in rat. Invest Ophthalmol Vis Sci 2013;54:5012–9.
Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F . The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev 2009;62:99–108.
Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ . An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res 2009;37:4587–602.
Kaelin WG Jr, Ratcliffe PJ . Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393–402.
Mahon PC, Hirota K, Semenza GL . FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 2001;15:2675–86.
Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C . ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 2000;468:53–58.
Brugarolas J, Kaelin WG Jr . Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell 2004;6:7–10.
Lendahl U, Lee KL, Yang H, Poellinger L . Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet 2009;10:821–32.
Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol 2007;171:53–67.
Kaur C, Sivakumar V, Foulds WS, Luu CD, Ling EA . Cellular and vascular changes in the retina of neonatal rats after an acute exposure to hypoxia. Invest Ophthalmol Vis Sci 2009;50:5364–74.
Ergorul C, Ray A, Huang W, Wang DY, Ben Y, Cantuti-Castelvetri I, Grosskreutz CL . Hypoxia inducible factor-1 (HIF-1) and some HIF-1 target genes are elevated in experimental glaucoma. J Mol Neurosci 2010;42:183–191.
Lukiw WJ, Ottlecz A, Lambrou G, et al. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci 2003;44:4163–70.
Yokogami K, Yamashita S, Takeshima H . Hypoxia-induced decreases in SOCS3 increase STAT3 activation and upregulate VEGF gene expression. Brain Tumor Pathol 2013;30:135–43.
Greisen G . Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev 2005;81:423–8.
Moroz E, Carlin S, Dyomina K, et al. Real-time imaging of HIF-1alpha stabilization and degradation. PLoS One 2009;4:e5077.
Yang H, Feng GD, Liang Z, et al. In vitro beneficial activation of microglial cells by mechanically-injured astrocytes enhances the synthesis and secretion of BDNF through p38MAPK. Neurochem Int 2012;61:175–86.
Li L, Qu Y, Li J, Xiong Y, Mao M, Mu D . Relationship between HIF-1alpha expression and neuronal apoptosis in neonatal rats with hypoxia-ischemia brain injury. Brain Res 2007;1180:133–9.
Skappak C, Regush S, Cheung PY, Adamko DJ . Identifying hypoxia in a newborn piglet model using urinary NMR metabolomic profiling. PLoS One 2013;8:e65035.
Siddiq A, Aminova LR, Ratan RR . Prolyl 4-hydroxylase activity-responsive transcription factors: from hydroxylation to gene expression and neuroprotection. Front Biosci 2008;13:2875–87.
Wang H, Byfield G, Jiang Y, Smith GW, McCloskey M, Hartnett ME . VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating local erythropoietin expression. Am J Pathol 2012;180:1243–53.
Tirziu D, Jaba IM, Yu P, et al. Endothelial nuclear factor-κB-dependent regulation of arteriogenesis and branching. Circulation 2012;126:2589–600.
Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR . Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci 1999;11:4159–70.
Perkins ND . Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007;8:49–62.
Niemeyer G . Retinal research using the perfused mammalian eye. Prog Retin Eye Res 2001;20:289–318.
Poitry S, Poitry-Yamate C, Ueberfeld J, MacLeish PR, Tsacopoulos M . Mechanisms of glutamate metabolic signaling in retinal glial (Müller) cells. J Neurosci 2000;20:1809–21.
Poitry-Yamate CL, Poitry S, Tsacopoulos M . Lactate released by Müller glial cells is metabolized by photoreceptors from mammalian retina. J Neurosci 1995;15:5179–91.
Blom J, Giove TJ, Pong WW, Blute TA, Eldred WD . Evidence for a functional adrenomedullin signaling pathway in the mouse retina. Mol Vis 2012;18:1339–53.
Kato H, Shichiri M, Marumo F, Hirata Y . Adrenomedullin as an autocrine/paracrine apoptosis survival factor for rat endothelial cells. Endocrinology 1997;138:2615–20.
Yuda K, Takahashi H, Inoue T, et al. Adrenomedullin inhibits choroidal neovascularization via CCL2 in the retinal pigment epithelium. Am J Pathol 2012;181:1464–72.
Fujita M, Kuwaki T, Ando K, Fujita T . Sympatho-inhibitory action of endogenous adrenomedullin through inhibition of oxidative stress in the brain. Hypertension 2005;45:1165–72.
Chaung WW, Wu R, Ji Y, et al. Peripheral administration of human adrenomedullin and its binding protein attenuates stroke-induced apoptosis and brain injury in rats. Mol Med 2011;17:1075–83.
Solberg R, Enot D, Deigner HP, et al. Metabolomic analyses of plasma reveals new insights into asphyxia and resuscitation in pigs. PLoS One 2010;5:e9606.
Enomoto M, Gosal K, Cubells E, et al. Sex-dependent changes in the pulmonary vasoconstriction potential of newborn rats following short-term oxygen exposure. Pediatr Res 2012;72:468–78.
Czok R, Lamprecht W . Pyruvate, Phosphoenolpyruvate and D-glycerate in Methods of Enzymatic Analysis. In Bergmeyer H, Gawehn K, eds. Methods of Enzymatic Analysis. Verlag Chemie: Weinheim Germany, 1974:1446–51.
Schmittgen TD, Livak KJ . Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101–8.
Acknowledgements
We would like to express our utmost gratitude to Sylvain Chemtob (Department of Pediatrics and Department of Pharmacology, Research Center-CHU Ste-Justine, Montréal, Quebec, Canada) for his intellectual and conceptual contribution to the hypothesis from which this manuscript originated, and to Frank van Bel (Division of Neonatology, Wilhelmina Children’s Hospital/University Medical Center Utrecht, The Netherlands) for his critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
(TIFF 2004 kb)
Supplementary Tables
(DOC 111 kb)
Supplementary Methods
(DOC 25 kb)
Rights and permissions
About this article
Cite this article
Arduini, A., Escobar, J., Vento, M. et al. Metabolic adaptation and neuroprotection differ in the retina and choroid in a piglet model of acute postnatal hypoxia. Pediatr Res 76, 127–134 (2014). https://doi.org/10.1038/pr.2014.70
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2014.70
This article is cited by
-
Early oxygen levels contribute to brain injury in extremely preterm infants
Pediatric Research (2021)
-
Early postnatal illness severity scores predict neurodevelopmental impairments at 10 years of age in children born extremely preterm
Journal of Perinatology (2017)