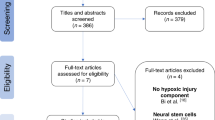
Abstract
Background:
Hypoxic–ischemic encephalopathy (HIE) is a major cause of morbidity in survivors. Therapeutic hypothermia (TH) is the only available intervention, but the protection is incomplete. Preclinical studies of HIE/TH in the rodent have relied on the postnatal day (P) 7 rat whose brain approximates a 32–36 wk gestation infant, less relevant for these studies. We propose that HIE and TH in the term-equivalent P10 rat will be more translational.
Methods:
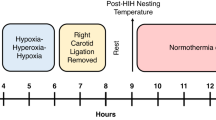
P10-11 rat pups were subjected to unilateral hypoxia–ischemia (HI) and 4 h recovery in normothermic (N) or hypothermic (TH) conditions. Brain damage was assessed longitudinally at 24 h, 2 wk, and 12 wk. Motor function was assessed with the beam walk; recognition memory was measured by novel object recognition.
Results:
Neuroprotection with TH was apparent at 2 and 12 wk in both moderately and severely damaged animals. TH improved motor function in moderate, but not severe, damage. Impaired object recognition occurred with severe damage with no evidence of protection of TH.
Conclusion:
This adaptation of the immature rat model of HI provides a reproducible platform to further study HIE/TH in which individual animals are followed up longitudinally to provide a useful translational preclinical model.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wu YW, March WM, Croen LA, Grether JK, Escobar GJ, Newman TB. Perinatal stroke in children with motor impairment: a population-based study. Pediatrics 2004;114:612–9.
Vannucci RC. Hypoxic-ischemic encephalopathy. Am J Perinatol 2000;17:113–20.
Gonzalez FF, Ferriero DM. Therapeutics for neonatal brain injury. Pharmacol Ther 2008;120:43–53.
Shankaran S, Pappas A, McDonald SA, et al.; Eunice Kennedy Shriver NICHD Neonatal Research Network. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012;366:2085–92.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Azzopardi DV, Strohm B, Edwards AD, et al.; TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58.
Thoresen M, Penrice J, Lorek A, et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 1995;37:667–70.
Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997;99:248–56.
Bona E, Hagberg H, Løberg EM, Bågenholm R, Thoresen M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res 1998;43:738–45.
Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981;9:131–41.
Patel SD, Pierce L, Ciardiello AJ, Vannucci SJ. Neonatal encephalopathy: pre-clinical studies in neuroprotection. Biochem Soc Trans 2014;42:564–8.
Goldstein LB. Model of recovery of locomotor ability after sensorimotor cortex injury in rats. ILAR J 2003;44:125–9.
Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 1988;31:47–59.
Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 2012;13:93–110.
Yager J, Towfighi J, Vannucci RC. Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 1993;34:525–9.
Thoresen M, Bågenholm R, Løberg EM, Apricena F, Kjellmer I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed 1996;74:F3–9.
Fang AY, Gonzalez FF, Sheldon RA, Ferriero DM. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia-ischemia. Pediatr Res 2013;73:12–7.
Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke 2012;43:3364–70.
Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke 2004;35:1460–5.
Barks JD, Liu YQ, Shangguan Y, Silverstein FS. Phenobarbital augments hypothermic neuroprotection. Pediatr Res 2010;67:532–7.
Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev 1991;26:61–7.
Tucker AM, Aquilina K, Chakkarapani E, Hobbs CE, Thoresen M. Development of amplitude-integrated electroencephalography and interburst interval in the rat. Pediatr Res 2009;65:62–6.
Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res Dev Brain Res 1997;100:149–60.
Kasdorf E, Engel M, Heier L, Perlman JM. Therapeutic hypothermia in neonates and selective hippocampal injury on diffusion-weighted magnetic resonance imaging. Pediatr Neurol 2014;51:104–8.
Azzopardi D, Strohm B, Edwards AD, et al.; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed 2009;94:F260–4.
Papile LA, Baley JE, Benitz W, et al. Hypothermia and neonatal encephalopathy. Pediatrics 2014;133:1146–50.
Thoresen M, Bågenholm R, Løberg EM, Apriccna F. The stress of being restrained reduces brain damage after a hypoxic-ischaemic insult in the 7-day-old rat. Neuroreport 1996;7:481–4.
Laptook A, Tyson J, Shankaran S, et al.; National Institute of Child Health and Human Development Neonatal Research Network. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics 2008;122:491–9.
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000;39:777–87.
Alvarado MC, Bachevalier J. Revisiting the maturation of medial temporal lobe memory functions in primates. Learn Mem 2000;7:244–56.
Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci 2011;31:10721–31.
Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 2004;24:5901–8.
Reger ML, Hovda DA, Giza CC. Ontogeny of rat recognition memory measured by the novel object recognition task. Dev Psychobiol 2009;51:672–8.
Rojas JJ, Deniz BF, Miguel PM, et al. Effects of daily environmental enrichment on behavior and dendritic spine density in hippocampus following neonatal hypoxia-ischemia in the rat. Exp Neurol 2013;241:25–33.
Davis AR, Shear DA, Chen Z, Lu XC, Tortella FC. A comparison of two cognitive test paradigms in a penetrating brain injury model. J Neurosci Methods 2010;189:84–7.
Albasser MM, Davies M, Futter JE, Aggleton JP. Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: Effects of varying the lesion extent and the duration of the sample period. Behav Neurosci 2009;123:115–24.
Rutherford MA, Azzopardi D, Whitelaw A, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 2005;116:1001–6.
Rutherford M, Pennock J, Schwieso J, Cowan F, Dubowitz L. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed 1996;75:F145–51.
Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143–9.
Vannucci SJ, Seaman LB, Vannucci RC. Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J Cereb Blood Flow Metab 1996;16:77–81.
Acknowledgements
We thank Jeffrey Perlman for support of these studies and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
(TIFF 332 kb)
Supplementary Figure S2
(TIFF 1634 kb)
Supplementary Figure S3
(TIFF 691 kb)
Supplementary Figure S4
(TIFF 378 kb)
Supplementary Figure S5
(TIFF 303 kb)
Rights and permissions
About this article
Cite this article
Patel, S., Pierce, L., Ciardiello, A. et al. Therapeutic hypothermia and hypoxia–ischemia in the term-equivalent neonatal rat: characterization of a translational preclinical model. Pediatr Res 78, 264–271 (2015). https://doi.org/10.1038/pr.2015.100
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.100
This article is cited by
-
TrkB-mediated sustained neuroprotection is sex-specific and \(\text{ER}\alpha\)-dependent in adult mice following neonatal hypoxia ischemia
Biology of Sex Differences (2024)
-
Rectal temperature after hypoxia-ischemia predicts white matter and cortical pathology in the near-term ferret
Pediatric Research (2024)
-
Systemic administration of clinical-grade multilineage-differentiating stress-enduring cells ameliorates hypoxic–ischemic brain injury in neonatal rats
Scientific Reports (2023)
-
Azithromycin reduces inflammation-amplified hypoxic–ischemic brain injury in neonatal rats
Pediatric Research (2022)
-
Neonatal hypoxia ischemia redistributes L1 cell adhesion molecule into rat cerebellar lipid rafts
Pediatric Research (2022)