Abstract
Background:
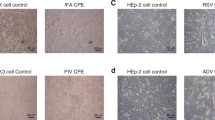
Viral culture plaque morphology in human cell lines are markers for growth capability and cytopathic effect, and have been used to assess viral fitness and select preattenuation candidates for live viral vaccines. We classified respiratory syncytial virus (RSV) plaque morphology and analyzed the relationship between plaque morphology as compared to subgroup, viral load and clinical severity of infection in infants and children.
Methods:
We obtained respiratory secretions from 149 RSV-infected children. Plaque morphology and viral load was assessed within the first culture passage in HEp-2 cells. Viral load was measured by polymerase chain reaction (PCR), as was RSV subgroup. Disease severity was determined by hospitalization, length of stay, intensive care requirement, and respiratory failure.
Results:
Plaque morphology varied between individual subjects; however, similar results were observed among viruses collected from upper and lower respiratory tracts of the same subject. Significant differences in plaque morphology were observed between RSV subgroups. No correlations were found among plaque morphology and viral load. Plaque morphology did not correlate with disease severity.
Conclusion:
Plaque morphology measures parameters that are viral-specific and independent of the human host. Morphologies vary between patients and are related to RSV subgroup. In HEp-2 cells, RSV plaque morphology appears unrelated to disease severity in RSV-infected children.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
El Saleeby CM, Li R, Somes GW, Dahmer MK, Quasney MW, DeVincenzo JP. Surfactant protein A2 polymorphisms and disease severity in a respiratory syncytial virus-infected population. J Pediatr 2010;156:409–14.
Miyairi I, DeVincenzo JP. Human genetic factors and respiratory syncytial virus disease severity. Clin Microbiol Rev 2008;21:686–703.
Thomsen SF, Stensballe LG, Skytthe A, Kyvik KO, Backer V, Bisgaard H. Increased concordance of severe respiratory syncytial virus infection in identical twins. Pediatrics 2008;121:493–6.
Fraser C, Lythgoe K, Leventhal GE, et al. Virulence and pathogenesis of HIV-1 infection: an evolutionary perspective. Science 2014;343:1243727.
Hakami A, Ali A, Hakami A. Effects of hepatitis B virus mutations on its replication and liver disease severity. Open Virol J 2013;7:12–8.
Goka EA, Vallely PJ, Mutton KJ, Klapper PE. Mutations associated with severity of the pandemic influenza A(H1N1)pdm09 in humans: a systematic review and meta-analysis of epidemiological evidence. Arch Virol 2014;159:3167–83.
Heikkinen T, Waris M, Ruuskanen O, Putto-Laurila A, Mertsola J. Incidence of acute otitis media associated with group A and B respiratory syncytial virus infections. Acta Paediatr 1995;84:419–23.
Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis 1997;175:814–20.
Kneyber MC, Brandenburg AH, Rothbarth PH, de Groot R, Ott A, van Steensel-Moll HA. Relationship between clinical severity of respiratory syncytial virus infection and subtype. Arch Dis Child 1996;75:137–40.
McIntosh ED, De Silva LM, Oates RK. Clinical severity of respiratory syncytial virus group A and B infection in Sydney, Australia. Pediatr Infect Dis J 1993;12:815–9.
Martinello RA, Chen MD, Weibel C, Kahn JS. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis 2002;186:839–42.
El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 2011;204:996–1002.
DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis 2005;191:1861–8.
Devincenzo JP. Natural infection of infants with respiratory syncytial virus subgroups A and B: a study of frequency, disease severity, and viral load. Pediatr Res 2004;56:914–7.
Dick JW, Rosenthal KS. A block in glycoprotein processing correlates with small plaque morphology and virion targetting to cell-cell junctions for an oral and an anal strain of herpes simplex virus type-1. Arch Virol 1995;140:2163–81.
Goel N, Mao H, Rong Q, Docherty JJ, Zimmerman D, Rosenthal KS. The ability of an HSV strain to initiate zosteriform spread correlates with its neuroinvasive disease potential. Arch Virol 2002;147:763–73.
Mao H, Rosenthal KS. Strain-dependent structural variants of herpes simplex virus type 1 ICP34.5 determine viral plaque size, efficiency of glycoprotein processing, and viral release and neuroinvasive disease potential. J Virol 2003;77:3409–17.
Jia Y, Moudy RM, Dupuis AP 2nd, et al. Characterization of a small plaque variant of West Nile virus isolated in New York in 2000. Virology 2007;367:339–47.
Davis CT, Beasley DW, Guzman H, et al. Emergence of attenuated West Nile virus variants in Texas, 2003. Virology 2004;330:342–50.
Blaney JE Jr, Johnson DH, Manipon GG, et al. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology 2002;300:125–39.
Rumyantsev AA, Murphy BR, Pletnev AG. A tick-borne Langat virus mutant that is temperature sensitive and host range restricted in neuroblastoma cells and lacks neuroinvasiveness for immunodeficient mice. J Virol 2006;80:1427–39.
Nickells M, Chambers TJ. Neuroadapted yellow fever virus 17D: determinants in the envelope protein govern neuroinvasiveness for SCID mice. J Virol 2003;77:12232–42.
Vlaycheva LA, Chambers TJ. Neuroblastoma cell-adapted yellow fever 17D virus: characterization of a viral variant associated with persistent infection and decreased virus spread. J Virol 2002;76:6172–84.
Vlaycheva L, Nickells M, Droll DA, Chambers TJ. Neuroblastoma cell-adapted yellow fever virus: mutagenesis of the E protein locus involved in persistent infection and its effects on virus penetration and spread. J Gen Virol 2005;86(Pt 2):413–21.
Holder BP, Simon P, Liao LE, et al. Assessing the in vitro fitness of an oseltamivir-resistant seasonal A/H1N1 influenza strain using a mathematical model. PLoS One 2011;6:e14767.
DeVincenzo JP, Wilkinson T, Vaishnaw A, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 2010;182:1305–14.
Buckingham SC, Bush AJ, Devincenzo JP. Nasal quantity of respiratory syncytical virus correlates with disease severity in hospitalized infants. Pediatr Infect Dis J 2000;19:113–7.
Grad YH, Newman R, Zody M, et al. Within-host whole-genome deep sequencing and diversity analysis of human respiratory syncytial virus infection reveals dynamics of genomic diversity in the absence and presence of immune pressure. J Virol 2014;88:7286–93.
Bower JR, Mao H, Durishin C, et al. Intrastrain variants of herpes simplex virus type 1 isolated from a neonate with fatal disseminated infection differ in the ICP34.5 gene, glycoprotein processing, and neuroinvasiveness. J Virol 1999;73:3843–53.
Jafri HS, Wu X, Makari D, Henrickson KJ. Distribution of respiratory syncytial virus subtypes A and B among infants presenting to the emergency department with lower respiratory tract infection or apnea. Pediatr Infect Dis J 2013;32:335–40.
Scull MA, Gillim-Ross L, Santos C, et al. Avian Influenza virus glycoproteins restrict virus replication and spread through human airway epithelium at temperatures of the proximal airways. PLoS Pathog 2009;5:e1000424.
Crowe JE Jr, Collins PL, London WT, Chanock RM, Murphy BR. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine 1993;11:1395–404.
Firestone CY, Whitehead SS, Collins PL, Murphy BR, Crowe JE Jr . Nucleotide sequence analysis of the respiratory syncytial virus subgroup A cold-passaged (cp) temperature sensitive (ts) cpts-248/404 live attenuated virus vaccine candidate. Virology 1996;225:419–22.
Juhasz K, Whitehead SS, Bui PT, et al. The temperature-sensitive (ts) phenotype of a cold-passaged (cp) live attenuated respiratory syncytial virus vaccine candidate, designated cpts530, results from a single amino acid substitution in the L protein. J Virol 1997;71:5814–9.
Karron RA, Wright PF, Crowe JE Jr, et al. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J Infect Dis 1997;176:1428–36.
Zhou B, Li Y, Speer SD, Subba A, Lin X, Wentworth DE. Engineering temperature sensitive live attenuated influenza vaccines from emerging viruses. Vaccine 2012;30:3691–702.
Perkins SM, Webb DL, Torrance SA, et al. Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol 2005;43:2356–62.
Acknowledgements
The authors thank April Sullivan for her technical help in the laboratory and Andrea Patters for her assistance with the preparation of the manuscript. We thank the parents and caregivers of the participating infants and children, without whose altruism the advancement towards therapy and prevention of RSV would be impossible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YI., Murphy, R., Majumdar, S. et al. Relating plaque morphology to respiratory syncytial virus subgroup, viral load, and disease severity in children. Pediatr Res 78, 380–388 (2015). https://doi.org/10.1038/pr.2015.122
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.122
This article is cited by
-
Reversible disruption of XPO1-mediated nuclear export inhibits respiratory syncytial virus (RSV) replication
Scientific Reports (2021)
-
A Virological and Phylogenetic Analysis of the Emergence of New Clades of Respiratory Syncytial Virus
Scientific Reports (2017)