Abstract
Background:
Near-term brain structure was examined in preterm infants in relation to neurodevelopment. We hypothesized that near-term macrostructural brain abnormalities identified using conventional magnetic resonance imaging (MRI), and white matter (WM) microstructure detected using diffusion tensor imaging (DTI), would correlate with lower cognitive and motor development and slower, less-stable gait at 18–22 mo of age.
Methods:
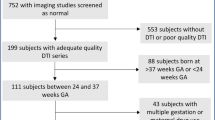
One hundred and two very-low-birth-weight preterm infants (≤1,500 g birth weight; ≤32 wk gestational age) were recruited prior to routine near-term brain MRI at 36.6 ± 1.8 wk postmenstrual age. Cerebellar and WM macrostructure was assessed on conventional structural MRI. DTI was obtained in 66 out of 102 and WM microstructure was assessed using fractional anisotropy and mean diffusivity (MD) in six subcortical brain regions defined by DiffeoMap neonatal atlas. Neurodevelopment was assessed with Bayley-Scales-of-Infant-Toddler-Development, 3rd-Edition (BSID-III); gait was assessed using an instrumented mat.
Results:
Neonates with cerebellar abnormalities identified using MRI demonstrated lower mean BSID-III cognitive composite scores (89.0 ± 10.1 vs. 97.8 ± 12.4; P = 0.002) at 18–22 mo. Neonates with higher DTI-derived left posterior limb of internal capsule (PLIC) MD demonstrated lower cognitive and motor composite scores (r = −0.368; P = 0.004; r = −0.354; P = 0.006) at 18–22 mo; neonates with higher genu MD demonstrated slower gait velocity (r = −0.374; P = 0.007). Multivariate linear regression significantly predicted cognitive (adjusted r2 = 0.247; P = 0.002) and motor score (adjusted r2 = 0.131; P = 0.017).
Conclusion:
Near-term cerebellar macrostructure and PLIC and genu microstructure were predictive of early neurodevelopment and gait.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Moore T, Hennessy EM, Myles J, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 2012;345:e7961.
Spittle AJ, Cheong J, Doyle LW, et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev Med Child Neurol 2011;53:1000–6.
Litt J, Taylor HG, Klein N, Hack M. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil 2005;38:130–41.
Rose J, Butler EE, Lamont LE, Barnes PD, Atlas SW, Stevenson DK. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol 2009;51:526–35.
Woodward LJ, Clark CA, Bora S, Inder TE. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One 2012;7:e51879.
Maulik PK, Darmstadt GL. Community-based interventions to optimize early childhood development in low resource settings. J Perinatol 2009;29:531–42.
Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci 2009;32:496–505.
Hintz SR, Barnes PD, Bulas D, et al.; SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics 2015;135:e32–42.
Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol 2003;24:1646–53.
Rose J, Mirmiran M, Butler EE, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol 2007;49:745–50.
De Bruïne FT, Van Wezel-Meijler G, Leijser LM, et al. Tractography of white-matter tracts in very preterm infants: a 2-year follow-up study. Dev Med Child Neurol 2013;55:427–33.
Dubois J, Dehaene-Lambertz G, Perrin M, et al. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp 2008;29:14–27.
Oishi K, Faria AV, Yoshida S, Chang L, Mori S. Quantitative evaluation of brain development using anatomical MRI and diffusion tensor imaging. Int J Dev Neurosci 2013;31:512–24.
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–48.
Chen Y, An H, Zhu H, et al. Longitudinal regression analysis of spatial-temporal growth patterns of geometrical diffusion measures in early postnatal brain development with diffusion tensor imaging. Neuroimage 2011;58:993–1005.
Rose J, Vassar R, Cahill-Rowley K, Guzman XS, Stevenson DK, Barnea-Goraly N. Brain microstructural development at near-term age in very-low-birth-weight preterm infants: an atlas-based diffusion imaging study. Neuroimage 2014;86:244–56.
Hüppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 2006;11:489–97.
Volpe JJ. The encephalopathy of prematurity–brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol 2009;16:167–78.
van Vliet EO, de Kieviet JF, Oosterlaan J, van Elburg RM. Perinatal infections and neurodevelopmental outcome in very preterm and very low-birth-weight infants: a meta-analysis. JAMA Pediatr 2013;167:662–8.
Mitha A, Foix-L’Hélias L, Arnaud C, et al.; EPIPAGE Study Group. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics 2013;132:e372–80.
Rose J, Vassar R, Cahill-Rowley K, et al. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. Neuroimage Clin 2014;5:169–77.
Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am J Neuroradiol 2011;32:2005–10.
Horsch S, Skiöld B, Hallberg B, et al. Cranial ultrasound and MRI at term age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2010;95:F310–4.
Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol 2009;24:1085–104.
Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ. Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex 2014;24:728–36.
Van Kooij BJ, Benders MJ, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol 2012;54:260–6.
Goergen SK, Ang H, Wong F, et al. Early MRI in term infants with perinatal hypoxic-ischaemic brain injury: interobserver agreement and MRI predictors of outcome at 2 years. Clin Radiol 2014;69:72–81.
Kersbergen KJ, Leemans A, Groenendaal F, et al. Microstructural brain development between 30 and 40 weeks corrected age in a longitudinal cohort of extremely preterm infants. Neuroimage 2014;103:214–24.
Provenzale JM, Isaacson J, Chen S. Progression of corpus callosum diffusion-tensor imaging values during a period of signal changes consistent with myelination. AJR Am J Roentgenol 2012;198:1403–8.
Vassar RL, Barnea-Goraly N, Rose J. Identification of neonatal white matter on DTI: influence of more inclusive thresholds for atlas segmentation. PLoS One 2014;9:e115426.
Caillé S, Sauerwein HC, Schiavetto A, Villemure JG, Lassonde M. Sensory and motor interhemispheric integration after section of different portions of the anterior corpus callosum in nonepileptic patients. Neurosurgery 2005;57:50–9; discussion 50–9.
Counsell SJ, Edwards AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 2008;131(Pt 12):3201–8.
Thompson DK, Inder TE, Faggian N, et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 2012;59:3571–81.
van Kooij BJ, van Pul C, Benders MJ, van Haastert IC, de Vries LS, Groenendaal F. Fiber tracking at term displays gender differences regarding cognitive and motor outcome at 2 years of age in preterm infants. Pediatr Res 2011;70:626–32.
Beaino G, Khoshnood B, Kaminski M, et al.; EPIPAGE Study Group. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol 2010;52:e119–25.
Yakovlev PI, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, ed. Regional Development of the Brain in Early Life. Oxford: Blackwell, 1967:3–70.
Liu XB, Shen Y, Plane JM, Deng W. Vulnerability of premyelinating oligodendrocytes to white-matter damage in neonatal brain injury. Neurosci Bull 2013;29:229–38.
Nolte J. The Human Brain: An Introduction to Its Functional Anatomy. 6th edn. Philadelphia, PA: Mosby/Elsevier, 2009.
Ball G, Boardman JP, Rueckert D, et al. The effect of preterm birth on thalamic and cortical development. Cereb Cortex 2012;22:1016–24.
Ball G, Boardman JP, Aljabar P, et al. The influence of preterm birth on the developing thalamocortical connectome. Cortex 2013;49:1711–21.
Kostović I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr 2010;99:1119–27.
Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 2013;81:2082–9.
Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–94.
Oishi K, Mori S, Donohue PK, et al. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage 2011;56:8–20.
Acknowledgements
We thank Alex Harris, John Tamaresis, Ron Cohen, Allan White, Elizabeth Loi, Megan Thompson, Anne DeBattista, Anne Fernald, and Heidi Feldman for valuable consultation and discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rose, J., Cahill-Rowley, K., Vassar, R. et al. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18–22 mo of age: an MRI and DTI study. Pediatr Res 78, 700–708 (2015). https://doi.org/10.1038/pr.2015.157
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.157
This article is cited by
-
Altered microstructure of the splenium of corpus callosum is associated with neurodevelopmental impairment in preterm infants with necrotizing enterocolitis
Italian Journal of Pediatrics (2022)
-
Nutrition and the developing brain: the road to optimizing early neurodevelopment: a systematic review
Pediatric Research (2020)
-
In vivo high-resolution diffusion tensor imaging of the developing neonatal rat cortex and its relationship to glial and dendritic maturation
Brain Structure and Function (2019)
-
Mild cerebellar injury does not significantly affect cerebral white matter microstructural organization and neurodevelopmental outcome in a contemporary cohort of preterm infants
Pediatric Research (2018)
-
Functional variation of SHP-2 promoter is associated with preterm birth and delayed myelination and motor development in preterm infants
Scientific Reports (2017)