Abstract
Background:
Necrotizing enterocolitis (NEC) is a life-threatening gastrointestinal disease in premature infants with high mortality and morbidity with uncertain pathogenesis. Recent research focused on the role of intraluminal bacteria and lipopolysaccharide (LPS). However, an additional role of viral agents in the pathogenesis of NEC has recently been postulated. We assessed the role of polyinosinic:polycytidylic acid (pIC) mimicking viral dsRNA in contributing to the development of NEC in neonatal mice.
Methods:
Four-d-old C57BL/6J pups were stressed by asphyxia and hypothermia twice daily. Animals were either fed by formula only (FO), formula containing LPS or pIC. After 72 h, mice were euthanized, intestines harvested, and the severity of NEC was assessed.
Results:
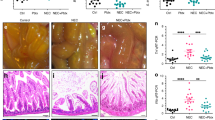
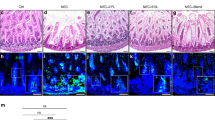
Breastfed mice showed no evidence of NEC. Very mild NEC-like lesions were observed in mice fed by FO. Supplementation of LPS or pIC to the formula led to increased intestinal tissue damage and inflammation compared with FO in a similar manner.
Conclusion:
Our study demonstrates the ability of viral factors to induce NEC in neonatal mice even in the absence of LPS. Furthermore, we present a new mouse model of pIC-induced NEC which may be used to obtain further mechanistic insights in the pathogenesis of this disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. N Engl J Med 1984;310:1093–103.
Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64.
Hackam DJ, Afrazi A, Good M, Sodhi CP. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin Dev Immunol 2013;2013:475415.
Herruzo R, Omeñaca F, García S, Diez J, Sánchez-Fauquier A. Identification of risk factors associated with nosocomial infection by rotavirus P4G2, in a neonatal unit of a tertiary-care hospital. Clin Microbiol Infect 2009;15:280–5.
Rotbart HA, Levin MJ, Yolken RH, Manchester DK, Jantzen J. An outbreak of rotavirus-associated neonatal necrotizing enterocolitis. J Pediatr 1983;103:454–9.
Sharma R, Garrison RD, Tepas JJ 3rd, et al. Rotavirus-associated necrotizing enterocolitis: an insight into a potentially preventable disease? J Pediatr Surg 2004;39:453–7.
Verdonk RC, Haagsma EB, Van Den Berg AP, et al. Inflammatory bowel disease after liver transplantation: a role for cytomegalovirus infection. Scand J Gastroenterol 2006;41:205–11.
Bagci S, Eis-Hübinger AM, Franz AR, et al. Detection of astrovirus in premature infants with necrotizing enterocolitis. Pediatr Infect Dis J 2008;27:347–50.
Clark B, McKendrick M. A review of viral gastroenteritis. Curr Opin Infect Dis 2004;17:461–9.
Lodha A, de Silva N, Petric M, Moore AM. Human torovirus: a new virus associated with neonatal necrotizing enterocolitis. Acta Paediatr 2005;94:1085–8.
Turcios-Ruiz RM, Axelrod P, St John K, et al. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr 2008;153:339–44.
Radulescu A, Zhang HY, Yu X, et al. Heparin-binding epidermal growth factor-like growth factor overexpression in transgenic mice increases resistance to necrotizing enterocolitis. J Pediatr Surg 2010;45:1933–9.
Tian R, Liu SX, Williams C, et al. Characterization of a necrotizing enterocolitis model in newborn mice. Int J Clin Exp Med 2010;3:293–302.
Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology 2009;137:221–30.
Cetin S, Ford HR, Sysko LR, et al. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem 2004;279:24592–600.
Leaphart CL, Cavallo J, Gribar SC, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol 2007;179:4808–20.
Sodhi CP, Neal MD, Siggers R, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012;143:708–718.e701–705.
Sodhi CP, Shi XH, Richardson WM, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology 2010;138:185–96.
Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 2005;14:145–51.
Gupta S, Morris JG Jr, Panigrahi P, Nataro JP, Glass RI, Gewolb IH. Endemic necrotizing enterocolitis: lack of association with a specific infectious agent. Pediatr Infect Dis J 1994;13:728–34.
Kosloske AM, Ulrich JA. A bacteriologic basis for the clinical presentations of necrotizing enterocolitis. J Pediatr Surg 1980;15:558–64.
Mollitt DL, Tepas JJ 3rd, Talbert JL. The microbiology of neonatal peritonitis. Arch Surg 1988;123:176–9.
Bagci S, Eis-Hübinger AM, Yassin AF, et al. Clinical characteristics of viral intestinal infection in preterm and term neonates. Eur J Clin Microbiol Infect Dis 2010;29:1079–84.
Dennehy PH. Transmission of rotavirus and other enteric pathogens in the home. Pediatr Infect Dis J 2000;19:Suppl 10 ::S103–5.
Broquet AH, Hirata Y, McAllister CS, Kagnoff MF. RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol 2011;186:1618–26.
Besch R, Poeck H, Hohenauer T, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 2009;119:2399–411.
McAllister CS, Lakhdari O, Pineton de Chambrun G, et al. TLR3, TRIF, and caspase 8 determine double-stranded RNA-induced epithelial cell death and survival in vivo. J Immunol 2013;190:418–27.
Pott J, Stockinger S, Torow N, et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog 2012;8:e1002670.
Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol 1994;14:1017–28.
Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 2013;6:498–510.
MohanKumar K, Kaza N, Jagadeeswaran R, et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am J Physiol Gastrointest Liver Physiol 2012;303:G93–102.
Pender SL, Braegger C, Gunther U, et al. Matrix metalloproteinases in necrotising enterocolitis. Pediatr Res 2003;54:160–4.
Gilchrist BF. Necrotizing Enterocolitis. 1st edn. Boca Raton, FL: CRC Press, 2000:15–76.
Maheshwari A, Kelly DR, Nicola T, et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011;140:242–53.
Leaphart CL, Qureshi F, Cetin S, et al. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 2007;132:2395–411.
Sodhi C, Richardson W, Gribar S, Hackam DJ. The development of animal models for the study of necrotizing enterocolitis. Dis Model Mech 2008;1:94–8.
McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm infant. Neoreviews 2011;12:e517–26.
Sangild PT. Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 2006;231:1695–711.
Caplan MS, Miller-Catchpole R, Kaup S, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology 1999;117:577–83.
Klemann C, Raveney BJ, Klemann AK, et al. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol 2009;174:2234–45.
Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A 2012;81:343–50.
Acknowledgements
We are grateful to Dorothee Viemann (research group of experimental neonatology) for her excellent theoretical and technical assistance.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
(PDF 130 kb)
Supplementary Figure S2
(PDF 123 kb)
Supplementary Figure S3
(PDF 491 kb)
Rights and permissions
About this article
Cite this article
Ginzel, M., Yu, Y., Klemann, C. et al. The viral dsRNA analogue poly (I:C) induces necrotizing enterocolitis in neonatal mice. Pediatr Res 79, 596–602 (2016). https://doi.org/10.1038/pr.2015.261
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.261