Abstract
Background:
Extremely preterm (EP, <28 wk gestation) individuals have increased the risk of cognitive deficits compared with controls. The posterior cingulate region has an important role in cognitive function, but how this is affected by preterm birth is unknown. We aimed to compare brain metabolite ratios of neurons and cell membranes between EP 18-y olds and controls, and explore the association between metabolite ratios and cognitive outcomes.
Method:
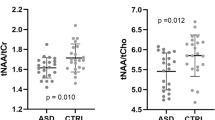
A regional cohort of 150 EP and 134 controls were recruited for the study. Cerebral metabolites were measured using proton magnetic resonance spectroscopy (MRS) obtained from a left posterior cingulate voxel. Total N-acetylaspartate (tNAA, neuronal marker)/total creatine (tCr), and total choline (tCho, cell membrane marker)/tCr ratios were compared between groups using linear regression. Metabolite ratios were correlated with tests of general intelligence (IQ), memory, and attention using linear or logistic regression.
Results:
Compared with controls, EP had lower tNAA/tCr (mean difference (95% CI) of −2.27% (−4.09, −0.45)) and tCho/tCr (mean difference (95% CI) of −11.11% (−20.37, −1.85)), all P = 0.02. Higher tCho/tCr correlated with better IQ in the EP group only; whereas higher tNAA/tCr ratios correlated with better scores in working memory and attention in both groups.
Conclusion:
EP birth is associated with long-term brain metabolite ratio alterations. This may underlie poorer cognitive performance in EP survivors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9.
Serenius F, Källén K, Blennow M, et al.; EXPRESS Group. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA 2013;309:1810–20.
Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ ; Victorian Infant Collaborative Study Group. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics 2013;131:e1053–61.
de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol 2012;54:313–23.
Cheong JL, Anderson PJ, Roberts G, et al. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PLoS One 2013;8:e77475.
Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol 2002;23:1445–56.
Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev 1989;13:23–31.
Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry 2005;10:900–19.
Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 2000;80:1107–213.
Pouwels PJ, Brockmann K, Kruse B, et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatr Res 1999;46:474–85.
Kendall GS, Melbourne A, Johnson S, et al. White matter NAA/Cho and Cho/Cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology 2014;271:230–8.
Xu D, Bonifacio SL, Charlton NN, et al. MR spectroscopy of normative premature newborns. J Magn Reson Imaging 2011;33:306–11.
Van Kooij BJ, Benders MJ, Anbeek P, Van Haastert IC, De Vries LS, Groenendaal F. Cerebellar volume and proton magnetic resonance spectroscopy at term, and neurodevelopment at 2 years of age in preterm infants. Dev Med Child Neurol 2012;54:260–6.
Gimenez M, Soria-Pastor S, Junque C, et al. Proton magnetic resonance spectroscopy reveals medial temporal metabolic abnormalities in adolescents with history of preterm birth. Pediatr Res 2008;64:572–7.
Bathen TF, Sjöbakk TE, Skranes J, et al. Cerebral metabolite differences in adolescents with low birth weight: assessment with in vivo proton MR spectroscopy. Pediatr Radiol 2006;36:802–9.
Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014;137(Pt 1):12–32.
Omizzolo C, Scratch SE, Stargatt R, et al. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory 2014;22:605–15.
Anderson PJ, De Luca CR, Hutchinson E, Spencer-Smith MM, Roberts G, Doyle LW ; Victorian Infant Collaborative Study Group. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol 2011;36:57–73.
Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res 2003;28:941–53.
Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med 1993;30:424–37.
Kantarci K. Proton MRS in mild cognitive impairment. J Magn Reson Imaging 2013;37:770–7.
van der Knaap MS, van der Grond J, Luyten PR, den Hollander JA, Nauta JJ, Valk J. 1H and 31P magnetic resonance spectroscopy of the brain in degenerative cerebral disorders. Ann Neurol 1992;31:202–11.
Stanley JA, Vemulapalli M, Nutche J, et al. Reduced N-acetyl-aspartate levels in schizophrenia patients with a younger onset age: a single-voxel 1H spectroscopy study. Schizophr Res 2007;93:23–32.
Tayebati SK, Amenta F. Choline-containing phospholipids: relevance to brain functional pathways. Clin Chem Lab Med 2013;51:513–21.
Kulak A, Duarte JM, Do KQ, Gruetter R. Neurochemical profile of the developing mouse cortex determined by in vivo 1H NMR spectroscopy at 14.1 T and the effect of recurrent anaesthesia. J Neurochem 2010;115:1466–77.
Augustine EM, Spielman DM, Barnes PD, et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatol 2008;28:611–8.
Schott JM, Frost C, MacManus DG, Ibrahim F, Waldman AD, Fox NC. Short echo time proton magnetic resonance spectroscopy in Alzheimer’s disease: a longitudinal multiple time point study. Brain 2010;133:3315–22.
Chiu PW, Mak HK, Yau KK, Chan Q, Chang RC, Chu LW. Metabolic changes in the anterior and posterior cingulate cortices of the normal aging brain: proton magnetic resonance spectroscopy study at 3 T. Age (Dordr) 2014;36:251–64.
The Victorian Infant Collaborative Study Group. Outcome at 2 years of children 23–27 weeks’ gestation born in Victoria in 1991–92. J Paediatr Child Health. 1997;33:161–5.
Doyle LW ; Victorian Infant Collaborative Study Group. Outcome at 5 years of age of children 23 to 27 weeks’ gestation: refining the prognosis. Pediatrics 2001;108:134–41.
Doyle LW, Anderson PJ ; Victorian Infant Collaborative Study Group. Improved neurosensory outcome at 8 years of age of extremely low birthweight children born in Victoria over three distinct eras. Arch Dis Child Fetal Neonatal Ed 2005;90:F484–8.
Cheong JL, Burnett AC, Lee KJ, et al. Association between Postnatal Dexamethasone for Treatment of Bronchopulmonary Dysplasia and Brain Volumes at Adolescence in Infants Born Very Preterm. J Pediatr. 2014;164:737–43.e1.
Wilson-Ching M, Molloy CS, Anderson VA, et al. Attention difficulties in a contemporary geographic cohort of adolescents born extremely preterm/extremely low birth weight. J Int Neuropsychol Soc 2013;19:1097–108.
Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001;14:260–4.
Provencher SW. LCModel User’s Manual. Version 6.3. San Diego, CA: LC Model Inc., 2013.
Wechsler D. Wechsler Intelligence Scale for Children. 3rd edn. San Antonio, TX: The Psychological Corporation, 1991.
Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The structure of normal human attention: The Test of Everyday Attention. J Int Neuropsychol Soc 1996;2:525–34.
Author information
Authors and Affiliations
Consortia
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Cheong, J., Bainbridge, A., Anderson, P. et al. Altered posterior cingulate brain metabolites and cognitive dysfunction in preterm adolescents. Pediatr Res 79, 716–722 (2016). https://doi.org/10.1038/pr.2015.272
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.272