Abstract
Background:
More pediatric drug trials are needed, but although specific pediatric regulations warrant safety, recruitment of children for these trials remains one of the main difficulties. Therefore, we investigated potential determining factors of nonparticipation in clinical research, in order to optimize research participation of children by recommending improved recruitment strategies.
Methods:
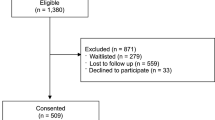
Between 1 January 2012 and 1 January 2014, we performed a prospective study among161 pediatric patients, aged 6 to 18 y, who were eligible for clinical research. We quantitatively analyzed the association of potential explanatory variables (e.g., age, cognitive development, experience, ethnicity) with nonparticipation and qualitatively analyzed interviews on reasons for nonparticipation.
Results:
Sixty percent of the children did not participate in the research project on offer (39% decided not to participate, 21% were indecisive). Lower age, less disease experience, and less complex research with lower risk were predictive for not participating. Time constraint and extra burden were expressed as decisive reasons for not participating.
Conclusions:
Strategies to optimize research participation should be aimed at younger children and their families, who are logistically challenged and unfamiliar with health care and research. Recommendations include informing pediatric patients and their families of the value of research; minimizing logistic burdens; and improving accessibility.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cuzzolin L, Atzei A, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf 2006;5:703–18.
Wendler D, Belsky L, Thompson KM, Emanuel EJ. Quantifying the federal minimal risk standard: implications for pediatric research without a prospect of direct benefit. JAMA 2005;294:826–32.
United States Department of Health and Human Services. Code of Federal Regulations. 45 CFR 46:408. Federal Policy for the Protection of Human Subjects (Subpart D). Washington, DC: United States Department of Health and Human Services, 2009.
Wendler D, Shah S. Should children decide whether they are enrolled in nonbeneficial research? Am J Bioeth 2003;3:1–7.
Hein IM, Troost PW, Lindeboom R, et al. Accuracy of the MacArthur competence assessment tool for clinical research (MacCAT-CR) for measuring children’s competence to consent to clinical research. JAMA Pediatr 2014;168:1147–53.
Vanhelst J, Hardy L, Bert D, et al. Effect of child health status on parents’ allowing children to participate in pediatric research. BMC Med Ethics 2013;14:7.
Oude Rengerink K. Embedding Trials in Evidence-Based Clinical Practice. Amsterdam: UvA-DARE, 2014.
Cox SM, McDonald M. Ethics is for human subjects too: participant perspectives on responsibility in health research. Soc Sci Med 2013;98:224–31.
Barakat LP, Patterson CA, Mondestin V, et al. Initial development of a questionnaire evaluating perceived benefits and barriers to pediatric clinical trials participation. Contemp Clin Trials 2013;34:218–26.
Wulf F, Krasuska M, Bullinger G. Determinants of decision-making and patient participation in peadiatrc clinical trials: a literature review. Open J Pediatr 2012;2:2–17.
Brody JL, Turner CW, Annett RD, Scherer DG, Dalen J. Predicting adolescent asthma research participation decisions from a structural equations model of protocol factors. J Adolesc Health 2012;51:252–8.
Miller VA, Baker JN, Leek AC, et al. Adolescent perspectives on phase I cancer research. Pediatr Blood Cancer 2013;60:873–8.
Unguru Y, Sill AM, Kamani N. The experiences of children enrolled in pediatric oncology research: implications for assent. Pediatrics 2010;125:e876–83.
Wendler D, Abdoler E, Wiener L, Grady C. Views of adolescents and parents on pediatric research without the potential for clinical benefit. Pediatrics 2012;130:692–9.
Reed JL, Thistlethwaite JM, Huppert JS. STI research: recruiting an unbiased sample. J Adolesc Health 2007;41:14–8.
Townsend A, Cox SM. Accessing health services through the back door: a qualitative interview study investigating reasons why people participate in health research in Canada. BMC Med Ethics 2013;14:40.
Hein IM, Troost PW, Lindeboom R, de Vries MC, Zwaan CM, Lindauer RJ. Assessing children’s competence to consent in research by a standardized tool: a validity study. BMC Pediatr 2012;12:156.
Alderson P. Competent children? Minors’ consent to health care treatment and research. Soc Sci Med 2007;65:2272–83.
Scherer DG, Annett RD, Brody JL. Ethical issues in adolescent and parent informed consent for pediatric asthma research participation. J Asthma 2007;44:489–96.
Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley, 2014.
Acknowledgements
We thank Marjolaine Oosterbeek, junior researcher at Impact, Arq Foundation, Diemen, for her assistance with MAXQDA. Many thanks are owed to Lotte Gelens, research assistant at Academic Medical Center, Amsterdam, for assisting in data collection.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Hein, I., Troost, P., de Vries, M. et al. Why do children decide not to participate in clinical research: a quantitative and qualitative study. Pediatr Res 78, 103–108 (2015). https://doi.org/10.1038/pr.2015.74
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.74
This article is cited by
-
Pediatric patient engagement in clinical care, research and intervention development: a scoping review
Journal of Patient-Reported Outcomes (2023)
-
Consent to research participation: understanding and motivation among German pupils
BMC Medical Ethics (2021)
-
Monitoring the recovery time of children after tonsillectomy using commercial activity trackers
European Journal of Pediatrics (2021)
-
Patient and caregiver engagement in research: factors that influence co-enrollment in research
Pediatric Rheumatology (2019)
-
The challenges of research participation by children
Pediatric Research (2015)