Abstract
Background:
Oxidative stress is associated with obesity while the evidence for the role of GH in pro- and antioxidation is inconclusive. This study investigates the relationships between growth hormone (GH), pro- and antioxidation in relation to obesity and puberty before and after an acute bout of exercise.
Methods:
In this case–control study, 76 healthy normal-weight and obese, prepubertal and pubertal boys underwent a blood sampling before and immediately after an aerobic exercise bout until exhaustion at 70% maximal oxygen consumption. Markers of prooxidation (thiobarbituric acid reactive substances (TBARS) and protein carbonyls (PCs)) and antioxidation (glutathione (GSH), oxidized glutathione disulfide (GSSG), GSH/GSSG ratio, glutathione peroxidase (GPX), catalase, and total antioxidant capacity (TAC)) and hormones (GH, insulin-like growth factor (IGF)-1, IGF-BP-3, luteinizing hormone, follicle-stimulating hormone, and testosterone) were measured.
Results:
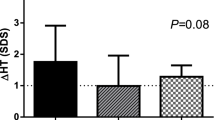
Baseline and postexercise TBARS and PCs were greater, while baseline GSH, GSH/GSSG ratio, GPX, and TAC were lower in obese than that in normal-weight participants. In all participants, waist was the best negative and positive predictor for postexercise GPX and TBARS, respectively. Baseline TAC was greater in pubertal than that in pre-pubertal participants. In all participants, baseline GH was the best negative predictor for postexercise PCs. Significant positive linear correlation exists between the exercise-associated GH, and GSSG increases in pubertal normal-weight boys.
Conclusions:
Higher prooxidation and lower antioxidation were observed in obese boys, while antioxidation improves with puberty and postexercise, paralleling GH accentuated secretion.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med 2009;8:1.
Germanou EI, Chatzinikolaou A, Malliou P, et al. Oxidative stress and inflammatory responses following an acute bout of isokinetic exercise in obese women with knee osteoarthritis. Knee 2013;20:581–90.
Margonis K, Fatouros IG, Jamurtas AZ, et al. Oxidative stress biomarkers responses to physical overtraining: implications for diagnosis. Free Radic Biol Med 2007;43:901–10.
Barbas I, Fatouros IG, Douroudos II, et al. Physiological and performance adaptations of elite Greco-Roman wrestlers during a one-day tournament. Eur J Appl Physiol 2011;111:1421–36.
Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis 2010;20:72–7.
Codoñer-Franch P, Tavárez-Alonso S, Murria-Estal R, Tortajada-Girbés M, Simó-Jordá R, Alonso-Iglesias E. Elevated advanced oxidation protein products (AOPPs) indicate metabolic risk in severely obese children. Nutr Metab Cardiovasc Dis 2012;22:237–43.
Atabek ME, Vatansev H, Erkul I. Oxidative stress in childhood obesity. J Pediatr Endocrinol Metab 2004;17:1063–8.
Burt Solorzano CM, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction 2010;140:399–410.
Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 2006;27:101–40.
Mohn A, Marzio D, Giannini C, Capanna R, Marcovecchio M, Chiarelli F. Alterations in the oxidant-antioxidant status in prepubertal children with growth hormone deficiency: effect of growth hormone replacement therapy. Clin Endocrinol (Oxf) 2005;63:537–42.
Evans LM, Davies JS, Anderson RA, et al. The effect of GH replacement therapy on endothelial function and oxidative stress in adult growth hormone deficiency. Eur J Endocrinol 2000;142:254–62.
Marin DP, Bolin AP, dos Santos Rde C, Curi R, Otton R. Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem Funct 2010;28:394–402.
Kogawa T, Nishimura M, Kurauchi S, Kashiwakura I. Characteristics of reactive oxygen metabolites in serum of early teenagers in Japan. Environ Health Prev Med 2012;17:364–70.
Benitez-Sillero Jde D, Perez-Navero JL, Tasset I, Guillen-Del Castillo M, Gil-Campos M, Tunez I. Influence of intense exercise on saliva glutathione in prepubescent and pubescent boys. Eur J Appl Physiol 2009;106:181–6.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–47.
Bouix O, Brun JF, Fédou C, et al. Plasma beta-endorphin, corticotrophin and growth hormone responses to exercise in pubertal and prepubertal children. Horm Metab Res 1994;26:195–9.
Luger A, Watschinger B, Deuster P, Svoboda T, Clodi M, Chrousos GP. Plasma growth hormone and prolactin responses to graded levels of acute exercise and to a lactate infusion. Neuroendocrinology 1992;56:112–7.
Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. Exercise and the stress system. Hormones (Athens) 2005;4:73–89.
Faienza MF, Francavilla R, Goffredo R, et al. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr 2012;78:158–64.
Mogri M, Dhindsa S, Quattrin T, Ghanim H, Dandona P. Testosterone concentrations in young pubertal and post-pubertal obese males. Clin Endocrinol (Oxf) 2013;78:593–9.
Vandewalle S, Taes Y, Fiers T, et al. Sex steroids in relation to sexual and skeletal maturation in obese male adolescents. J Clin Endocrinol Metab 2014;99:2977–85.
Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 2003;66:1499–503.
Ballerini MG, Ropelato MG, Domené HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (IGF)-IGF binding proteins axis. J Pediatr Endocrinol Metab 2004;17:749–57.
Romero CJ, Pine-Twaddell E, Sima DI, et al. Insulin-like growth factor 1 mediates negative feedback to somatotroph GH expression via POU1F1/CREB binding protein interactions. Mol Cell Biol 2012;32:4258–69.
Pomerants T, Tillmann V, Karelson K, Jürimäe J, Jürimäe T. Impact of acute exercise on bone turnover and growth hormone/insulin-like growth factor axis in boys. J Sports Med Phys Fitness 2008;48:266–71.
Consitt LA, Wideman L, Hickey MS, Morrison RF. Phosphorylation of the JAK2-STAT5 pathway in response to acute aerobic exercise. Med Sci Sports Exerc 2008;40:1031–8.
Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 2009;30:152–77.
Gatti R, De Palo EF, Antonelli G, Spinella P. IGF-I/IGFBP system: metabolism outline and physical exercise. J Endocrinol Invest 2012;35:699–707.
Bianchi G, Bugianesi E, Ronchi M, Fabbri A, Zoli M, Marchesini G. Glutathione kinetics in normal man and in patients with liver cirrhosis. J Hepatol 1997;26:606–13.
Cholez E, Debuysscher V, Bourgeais J, et al. Evidence for a protective role of the STAT5 transcription factor against oxidative stress in human leukemic pre-B cells. Leukemia 2012;26:2390–7.
Guo S, Wharton W, Moseley P, Shi H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 2007;12:245–54.
Fukushima M, Okamoto Y, Katsumata H, et al. Growth hormone ameliorates adipose dysfunction during oxidative stress and inflammation and improves glucose tolerance in obese mice. Horm Metab Res 2014;46:656–62.
Chiotis D, Krikos X, Tsiftis G, Hatzisymeon M, Maniati-Christidi M, Dacou-Voutetaki A. Body mass index and prevalence of obesity in subjects of Hellenic origin aged 0–18 years living in the Athens area. Ann Clin Pediatr Unive Atheniensis 2004; 51:139–54.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–3.
Mavrakanas TA, Konsoula G, Patsonis I, Merkouris BP. Childhood obesity and elevated blood pressure in a rural population of northern Greece. Rural Remote Health 2009;9:1150.
Veldhuis JD, Pincus SM, Mitamura R, et al. Developmentally delimited emergence of more orderly luteinizing hormone and testosterone secretion during late prepuberty in boys. J Clin Endocrinol Metab 2001;86:80–9.
Albertsson-Wikland K, Rosberg S, Lannering B, Dunkel L, Selstam G, Norjavaara E. Twenty-four-hour profiles of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol levels: a semilongitudinal study throughout puberty in healthy boys. J Clin Endocrinol Metab 1997;82:541–9.
Kelly AS, Steinberger J, Olson TP, Dengel DR. In the absence of weight loss, exercise training does not improve adipokines or oxidative stress in overweight children. Metabolism 2007;56:1005–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paltoglou, G., Fatouros, I., Valsamakis, G. et al. Antioxidation improves in puberty in normal weight and obese boys, in positive association with exercise-stimulated growth hormone secretion. Pediatr Res 78, 158–164 (2015). https://doi.org/10.1038/pr.2015.85
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2015.85
This article is cited by
-
Association between biomarkers of redox status and cytokines with different patterns of habitual physical activity in eutrophic and overweight/obese preschoolers: multivariate analysis of a cross-sectional study
BMC Public Health (2023)
-
Endocrine responses of the stress system to different types of exercise
Reviews in Endocrine and Metabolic Disorders (2023)
-
Exercise-induced GH secretion is related to puberty
Journal of Endocrinological Investigation (2021)
-
In early pubertal boys, testosterone and LH are associated with improved anti-oxidation during an aerobic exercise bout
Endocrine (2019)
-
Chronic stress and body composition disorders: implications for health and disease
Hormones (2018)