Abstract
Background:
Disturbances in cerebral blood flow (CBF) and brain oxygenation (PbO2) are present early after pediatric cardiac arrest (CA). CBF-targeted therapies improved neurological outcome in our CA model. To assess the therapeutic window for CBF- and PbO2-targeted therapies, we propose to determine if CBF and PbO2 disturbances persist at 24 h after experimental pediatric CA.
Methods:
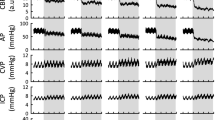
Regional CBF and PbO2 were measured at 24 h after asphyxial CA in immature rats (n = 26, 6–8/group) using arterial spin label MRI and tissue electrodes, respectively.
Results:
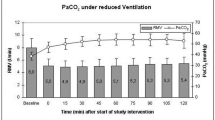
In all regions but the thalamus, CBF recovered to sham values by 24 h; thalamic CBF was >32% higher after CA vs. sham. PbO2 values at 24 h after CA in the cortex and thalamus were similar to shams in rats who received supplemental oxygen, however, on room air, cortical PbO2 was lower after CA vs. shams.
Conclusion:
CBF remains increased in the thalamus at 24 h after CA and PbO2 is decreased to hypoxic levels in cortex at 24 h after CA in rats who do not receive supplemental oxygen. Given the enduring disturbances in this model and the lack of routine CBF or PbO2 monitoring in patients, our data suggest the need for clinical correlation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Moler FW, Meert K, Donaldson AE, et al.; Pediatric Emergency Care Applied Research Network. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med 2009;37:2259–67.
Manole MD, Foley LM, Hitchens TK, et al. Magnetic resonance imaging assessment of regional cerebral blood flow after asphyxial cardiac arrest in immature rats. J Cereb Blood Flow Metab 2009;29:197–205.
Manole MD, Kochanek PM, Bayır H, et al. Brain tissue oxygen monitoring identifies cortical hypoxia and thalamic hyperoxia after experimental cardiac arrest in rats. Pediatr Res 2014;75:295–301.
Manole MD, Kochanek PM, Foley LM, et al. Polynitroxyl albumin and albumin therapy after pediatric asphyxial cardiac arrest: effects on cerebral blood flow and neurologic outcome. J Cereb Blood Flow Metab 2012;32:560–9.
Narotam PK, Morrison JF, Nathoo N. Brain tissue oxygen monitoring in traumatic brain injury and major trauma: outcome analysis of a brain tissue oxygen-directed therapy. J Neurosurg 2009;111:672–82.
Maloney-Wilensky E, Gracias V, Itkin A, et al. Brain tissue oxygen and outcome after severe traumatic brain injury: a systematic review. Crit Care Med 2009;37:2057–63.
Manole MD, Kochanek PM, Foley LM, et al. Polynitroxyl albumin and albumin therapy after pediatric asphyxial cardiac arrest: effects on cerebral blood flow and neurologic outcome. J Cereb Blood Flow Metab 2012;32:560–9.
Shaik JS, Poloyac SM, Kochanek PM, et al. 20-Hydroxyeicosatetraenoic acid inhibition by HET0016 offers neuroprotection, decreases edema, and increases cortical cerebral blood flow in a pediatric asphyxial cardiac arrest model in Rats. J Cereb Blood Flow Metab 2015;35:1757–63.
Choi SP, Park KN, Park HK, et al. Diffusion-weighted magnetic resonance imaging for predicting the clinical outcome of comatose survivors after cardiac arrest: a cohort study. Crit Care 2010;14:R17.
Radovsky A, Katz L, Ebmeyer U, Safar P. Ischemic neurons in rat brains after 6, 8, or 10 minutes of transient hypoxic ischemia. Toxicol Pathol 1997;25:500–5.
Böttiger BW, Schmitz B, Wiessner C, Vogel P, Hossmann KA. Neuronal stress response and neuronal cell damage after cardiocirculatory arrest in rats. J Cereb Blood Flow Metab 1998;18:1077–87.
Geocadin RG, Muthuswamy J, Sherman DL, Thakor NV, Hanley DF. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov Disord 2000;15 Suppl 1:14–21.
Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA 1984;81:4586–90.
McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature 2008;456:391–4.
McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 1997;20:185–215.
Hartings JA, Simons DJ. Inhibition suppresses transmission of tonic vibrissa-evoked activity in the rat ventrobasal thalamus. J Neurosci 2000;20:RC100.
Venkatesan A, Frucht S. Movement disorders after resuscitation from cardiac arrest. Neurol Clin 2006;24:123–32.
Shoykhet M, Simons DJ, Alexander H, Hosler C, Kochanek PM, Clark RS. Thalamocortical dysfunction and thalamic injury after asphyxial cardiac arrest in developing rats. J Neurosci 2012;32:4972–81.
Aravamuthan BR, Shoykhet M. Long-term increase in coherence between the basal ganglia and motor cortex after asphyxial cardiac arrest and resuscitation in developing rats. Pediatr Res 2015;78:371–9.
Goodman JC, Valadka AB, Gopinath SP, Uzura M, Robertson CS. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med 1999;27:1965–73.
Nordmark J, Rubertsson S, Mörtberg E, Nilsson P, Enblad P. Intracerebral monitoring in comatose patients treated with hypothermia after a cardiac arrest. Acta Anaesthesiol Scand 2009;53:289–98.
Hayashida K, Nishiyama K, Suzuki M, et al. Estimated cerebral oxyhemoglobin as a useful indicator of neuroprotection in patients with post-cardiac arrest syndrome: a prospective, multicenter observational study. Crit Care 2014;18:500.
Genbrugge C, Meex I, Boer W, et al. Increase in cerebral oxygenation during advanced life support in out-of-hospital patients is associated with return of spontaneous circulation. Crit Care 2015;19:112.
Bouglé A, Daviaud F, Bougouin W, et al. Determinants and significance of cerebral oximetry after cardiac arrest: A prospective cohort study. Resuscitation 2016;99:1–6.
Duggan M, McNamara PJ, Engelberts D, et al. Oxygen attenuates atelectasis-induced injury in the in vivo rat lung. Anesthesiology 2005;103:522–31.
Atkins JL, Johnson KB, Pearce FJ. Cardiovascular responses to oxygen inhalation after hemorrhage in anesthetized rats: hyperoxic vasoconstriction. Am J Physiol Heart Circ Physiol 2007;292:H776–85.
Jackson WF. Arteriolar oxygen reactivity: where is the sensor? Am J Physiol 1987;253(5 Pt 2):H1120–6.
Fink EL, Alexander H, Marco CD, et al. Experimental model of pediatric asphyxial cardiopulmonary arrest in rats. Pediatr Crit Care Med 2004;5:139–44.
Hendrich KS, Kochanek PM, Melick JA, et al. Cerebral perfusion during anesthesia with fentanyl, isoflurane, or pentobarbital in normal rats studied by arterial spin-labeled MRI. Magn Reson Med 2001;46:202–6.
Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med 1992;23:37–45.
Hendrich KS, Kochanek PM, Williams DS, Schiding JK, Marion DW, Ho C. Early perfusion after controlled cortical impact in rats: quantification by arterial spin-labeled MRI and the influence of spin-lattice relaxation time heterogeneity. Magn Reson Med 1999;42:673–81.
Zhang W, Williams DS, Koretsky AP. Measurement of rat brain perfusion by NMR using spin labeling of arterial water: in vivo determination of the degree of spin labeling. Magn Reson Med 1993;29:416–21.
Herscovitch P, Raichle ME. What is the correct value for the brain–blood partition coefficient for water? J Cereb Blood Flow Metab 1985;5:65–9.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Foley, L., Clark, R., Vazquez, A. et al. Enduring disturbances in regional cerebral blood flow and brain oxygenation at 24 h after asphyxial cardiac arrest in developing rats. Pediatr Res 81, 94–98 (2017). https://doi.org/10.1038/pr.2016.175
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.175
This article is cited by
-
Early Thalamocortical Reperfusion Leads to Neurologic Recovery in a Rodent Cardiac Arrest Model
Neurocritical Care (2022)
-
Neonatal cerebrovascular autoregulation
Pediatric Research (2018)