Abstract
Background:
Critically ill preterm and term neonates are at high risk for negative iron balance due to phlebotomy that occurs with frequent laboratory monitoring, and the high iron demand of rapid growth. Understanding the prioritization of iron between red blood cells (RBCs) and brain is important given iron’s role in neurodevelopment.
Methods:
Ten neonatal twin lamb pairs (n = 20) underwent regular phlebotomy for 11 d. Lambs were randomized to receive no iron or i.v. daily iron supplementation from 1 to 5 mg/kg. Serum hemoglobin concentration and reticulocyte count were assayed, iron balance calculated, and iron content of RBCs, liver, brain, muscle, and heart measured at autopsy.
Results:
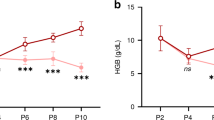
Among phlebotomized lambs: (i) liver iron concentration was directly related to net iron balance (r = 0.87; P < 0.001) and (ii) brain iron concentration was reduced as a function of net iron balance (r = 0.63) only after liver iron was depleted. In animals with negative iron balance, total RBC iron was maintained while brain iron concentration decreased as a percentage of the iron present in RBCs (r = −0.70; P < 0.01) and as a function of reticulocyte count (r = −0.63; P < 0.05).
Conclusion:
Phlebotomy-induced negative iron balance limits iron availability to the developing brain.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Neoreviews 2008;9:e520.
DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol 2005;47:525–31.
Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr 2010;156:377–81.
Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr 1996;126:693–701.
McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr Neurosci 2005;8:195–206.
Ortiz E, Pasquini JM, Thompson K, et al. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res 2004;77:681–9.
Kirpalani H, Whyte RK, Andersen C, et al. The premature infants in need of transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006;149:301–7.
Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 2005;115:1685–91.
McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol 2011;17:347–67.
Whyte RK, Kirpalani H, Asztalos EV, et al.; PINTOS Study Group. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics 2009;123:207–13.
Guillén U, Cummings JJ, Bell EF, et al. International survey of transfusion practices for extremely premature infants. Semin Perinatol 2012;36:244–7.
Urrechaga E, Borque L, Escanero JF. Biomarkers of hypochromia: the contemporary assessment of iron status and erythropoiesis. Biomed Res Int 2013;2013:603786.
Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr 1992;121:109–14.
Georgieff MK, Petry CD, Wobken JD, Oyer CE. Liver and brain iron deficiency in newborn infants with bilateral renal agenesis (Potter’s syndrome). Pediatr Pathol Lab Med 1996;16:509–19.
Kuzawa CW. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am J Hum Biol 2005;17:5–21.
Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr 2011;2:112–21.
Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier RA. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res 2004;55:1034–41.
Connor JR, Menzies SL, St Martin SM, Mufson EJ. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res 1990;27:595–611.
Georgieff MK, Schmidt RL, Mills MM, Radmer WJ, Widness JA. Fetal iron and cytochrome c status after intrauterine hypoxemia and erythropoietin administration. Am J Physiol 1992;262:R485–91.
de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res 2000;48:169–76.
Mash DC, Pablo J, Flynn DD, Efange SM, Weiner WJ. Characterization and distribution of transferrin receptors in the rat brain. J Neurochem 1990;55:1972–9.
Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol 2000;20:77–95.
Taylor EM, Morgan EH. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res 1990;55:35–42.
Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res 2002;68:761–75.
Cheah JH, Kim SF, Hester LD, et al. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron 2006;51:431–40.
Erecinska M, Cherian S, Silver IA. Energy metabolism in mammalian brain during development. Prog Neurobiol 2004;73:397–445.
Fretham SJ, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus 2012;22:1691–702.
Guiang SF 3rd, Widness JA, Flanagan KB, Schmidt RL, Radmer WJ, Georgieff MK. The relationship between fetal arterial oxygen saturation and heart and skeletal muscle myoglobin concentrations in the ovine fetus. J Dev Physiol 1993;19:99–104.
Fretham SJ, Carlson ES, Georgieff MK. Neuronal-specific iron deficiency dysregulates mammalian target of rapamycin signaling during hippocampal development in nonanemic genetic mouse models. J Nutr 2013;143:260–6.
Carlson ES, Stead JD, Neal CR, Petryk A, Georgieff MK. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus 2007;17:679–91.
Leonard WR, Robertson ML, Snodgrass JJ, Kuzawa CW. Metabolic correlates of hominid brain evolution. Comp Biochem Physiol A Mol Integr Physiol 2003;136:5–15.
Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol 1998;Suppl 27:177–209.
Iron fortification of infant formulas. American Academy of Pediatrics. Committee on Nutrition. Pediatrics 1999;104:119–23.
Fomon SJ, Nelson SE, Ziegler EE. Retention of iron by infants. Annu Rev Nutr 2000;20:273–90.
Oski FA. Iron deficiency in infancy and childhood. N Engl J Med 1993;329:190–3.
Mills RJ, Davies MW. Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database Syst Rev 2012;3:CD005095.
Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. J Pediatr 1996;129:680–7.
Shafir T, Angulo-Barroso R, Jing Y, Angelilli ML, Jacobson SW, Lozoff B. Iron deficiency and infant motor development. Early Hum Dev 2008;84:479–85.
Tamura T, Goldenberg RL, Hou J, et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr 2002;140:165–70.
Guiang SF 3rd, Georgieff MK, Lambert DJ, Schmidt RL, Widness JA. Intravenous iron supplementation effect on tissue iron and hemoproteins in chronically phlebotomized lambs. Am J Physiol 1997;273:R2124–31.
Acknowledgements
We acknowledge Robert L. Schmidt, Barbara Stewart, and Kathy Byers at the University of Iowa and Jane Wobken at the University of Minnesota for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamora, T., Guiang, S., Widness, J. et al. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res 79, 922–928 (2016). https://doi.org/10.1038/pr.2016.20
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.20
This article is cited by
-
New frontiers in neonatal red blood cell transfusion research
Journal of Perinatology (2023)
-
Hepcidin is a relevant iron status indicator in infancy: results from a randomized trial of early vs. delayed cord clamping
Pediatric Research (2021)
-
Evidence for communication of peripheral iron status to cerebrospinal fluid: clinical implications for therapeutic strategy
Fluids and Barriers of the CNS (2020)
-
Trends in reticulocyte hemoglobin equivalent values in critically ill neonates, stratified by gestational age
Journal of Perinatology (2019)
-
Effects of iron supplementation of low-birth-weight infants on cognition and behavior at 7 years: a randomized controlled trial
Pediatric Research (2018)