Abstract
Background:
Retinopathy of prematurity (ROP) is a potentially blinding, retinal neovascular disease. Systemic prolactin accesses the retina to regulate blood vessels. Prolactin is proangiogenic and can be cleaved to antiangiogenic vasoinhibins. We investigated whether circulating prolactin and vasoinhibins associate with incidence and progression of ROP.
Methods:
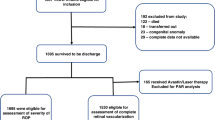
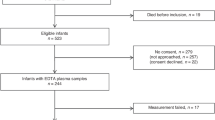
A prospective, longitudinal, case–control study covering postnatal weeks 1 to 9 measured serum prolactin, vasoinhibins, and vascular endothelial growth factor (VEGF) weekly in 90 premature infants diagnosed as ROP or control.
Results:
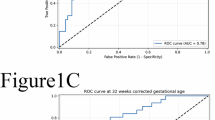
Prolactin levels were higher in ROP than in control patients before (106.2 ± 11.3 (SEM) vs. 64.7 ± 4.9 ng/ml, postnatal week 1) and during (120.6 ± 10 vs. 84.7 ± 7.5ng/ml, postnatal week 5) ROP diagnosis. Prolactin, but not gestational age, birth weight, Apgar score, sepsis, or ventilation time, correlated with ROP. The relative risk (RR) of developing ROP increased if Prolactin (PRL) levels were higher than thresholds of 80 ng/ml (RR = 1.55, 95% CI: 1.06–2.28), 100 ng/ml (RR = 1.63, 95% CI: 1.14–2.34), or 120 ng/ml (RR = 1.95, 95% CI: 1.41–2.68). Vasoinhibin levels were 39.7% higher (95% CI: 4.5–77.5) in the circulation of ROP than in control patients at postnatal week 1 and similar thereafter, whereas VEGF serum levels were always similar.
Conclusion:
High serum prolactin and vasoinhibin levels predict and may impact ROP progression.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Friddle K. Pathogenesis of retinopathy of prematurity: Does inflamation play a role? Newborn Infant Nurs Rev 2013; 13:161–165.
Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet 2013;382:1445–57.
Budd SJ, Hartnett ME. Increased angiogenic factors associated with peripheral avascular retina and intravitreous neovascularization: a model of retinopathy of prematurity. Arch Ophthalmol 2010;128:589–95.
Harder BC, von Baltz S, Jonas JB, Schlichtenbrede FC. Intravitreal low-dosage bevacizumab for retinopathy of prematurity. Acta Ophthalmol 2014;92:577–81.
Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 2009;116:1599–603.
Hellström A, Engström E, Hård AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003;112:1016–20.
Clapp C, Thebault S, Macotela Y, Moreno-Carranza B, Triebel J, Martínez de la Escalera G. Regulation of blood vessels by prolactin and vasoinhibins. Adv Exp Med Biol 2015;846:83–95.
Triebel J, Bertsch T, Bollheimer C, et al. Principles of the prolactin/vasoinhibin axis. Am J Physiol Regul Integr Comp Physiol 2015;309:R1193–203.
Dueñas Z, Torner L, Corbacho AM, et al. Inhibition of rat corneal angiogenesis by 16-kDa prolactin and by endogenous prolactin-like molecules. Invest Ophthalmol Vis Sci 1999;40:2498–505.
Aranda J, Rivera JC, Jeziorski MC, et al. Prolactins are natural inhibitors of angiogenesis in the retina. Invest Ophthalmol Vis Sci 2005;46:2947–53.
Arnold E, Rivera JC, Thebault S, et al. High levels of serum prolactin protect against diabetic retinopathy by increasing ocular vasoinhibins. Diabetes 2010;59:3192–7.
García C, Aranda J, Arnold E, et al. Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A-dependent eNOS inactivation. J Clin Invest 2008;118:2291–300.
Ramírez M, Wu Z, Moreno-Carranza B, et al. Vasoinhibin gene transfer by adenoassociated virus type 2 protects against VEGF- and diabetes-induced retinal vasopermeability. Invest Ophthalmol Vis Sci 2011;52:8944–50.
Pan H, Nguyen NQ, Yoshida H, et al. Molecular targeting of antiangiogenic factor 16K hPRL inhibits oxygen-induced retinopathy in mice. Invest Ophthalmol Vis Sci 2004;45:2413–9.
Dueñas Z, Rivera JC, Quiróz-Mercado H, et al. Prolactin in eyes of patients with retinopathy of prematurity: implications for vascular regression. Invest Ophthalmol Vis Sci 2004;45:2049–55.
Parker CR Jr, MacDonald PC, Guzick DS, Porter JC, Rosenfeld CR, Hauth JC. Prolactin levels in umbilical cord blood of human infants: relation to gestational age, maternal complications, and neonatal lung function. Am J Obstet Gynecol 1989;161:795–802.
González C, Parra A, Ramírez-Peredo J, et al. Elevated vasoinhibins may contribute to endothelial cell dysfunction and low birth weight in preeclampsia. Lab Invest 2007;87:1009–17.
Leaños-Miranda A, Márquez-Acosta J, Cárdenas-Mondragón GM, et al. Urinary prolactin as a reliable marker for preeclampsia, its severity, and the occurrence of adverse pregnancy outcomes. J Clin Endocrinol Metab 2008;93:2492–9.
Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600.
International Committee for the Classification of Retinopathy of P. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005; 123:991–999.
Piwnica D, Touraine P, Struman I, et al. Cathepsin D processes human prolactin into multiple 16K-like N-terminal fragments: study of their antiangiogenic properties and physiological relevance. Mol Endocrinol 2004;18:2522–42.
Flynn JT. Acute proliferative retrolental fibroplasia: multivariate risk analysis. Trans Am Ophthalmol Soc 1983;81:549–91.
Hellgren G, Löfqvist C, Hård AL, et al. Serum concentrations of vascular endothelial growth factor in relation to retinopathy of prematurity. Pediatr Res 2016;79:70–5.
Velez-Montoya R, Clapp C, Rivera JC, et al. Intraocular and systemic levels of vascular endothelial growth factor in advanced cases of retinopathy of prematurity. Clin Ophthalmol 2010;4:947–53.
Tyson JE, Hwang P, Guyda H, Friesen HG. Studies of prolactin secretion in human pregnancy. Am J Obstet Gynecol 1972;113:14–20.
Winters AJ, Colston C, MacDonald PC, Porter JC. Fetal plasma prolactin levels. J Clin Endocrinol Metab 1975;41:626–9.
Ben-Jonathan N, Munsick RA. Dopamine and prolactin in human pregnancy. J Clin Endocrinol Metab 1980;51:1019–25.
Guyda HJ, Friesen HG. Serum prolactin levels in humans from birth to adult life. Pediatr Res 1973;7:534–40.
Lucas A, Baker BA, Cole TJ. Plasma prolactin and clinical outcome in preterm infants. Arch Dis Child 1990;65:977–83.
Notari L, Miller A, Martínez A, et al. Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia. Invest Ophthalmol Vis Sci 2005;46:2736–47.
Zhang W, Yokota H, Xu Z, et al. Hyperoxia therapy of pre-proliferative ischemic retinopathy in a mouse model. Invest Ophthalmol Vis Sci 2011;52:6384–95.
Macotela Y, Aguilar MB, Guzmán-Morales J, et al. Matrix metalloproteases from chondrocytes generate an antiangiogenic 16 kDa prolactin. J Cell Sci 2006;119(Pt 9):1790–800.
Hayasaka S. Lysosomal enzymes in ocular tissues and diseases. Surv Ophthalmol 1983;27:245–58.
Chen YH, Chou HC, Lin ST, Chen YW, Lo YW, Chan HL. Effect of high glucose on secreted proteome in cultured retinal pigmented epithelium cells: its possible relevance to clinical diabetic retinopathy. J Proteomics 2012;77:111–28.
Clapp C, Sears PS, Russell DH, Richards J, Levay-Young BK, Nicoll CS. Biological and immunological characterization of cleaved and 16K forms of rat prolactin. Endocrinology 1988;122:2892–8.
O’Steen WK, Sundberg DK. Patterns of radioactivity in the eyes of rats after injection of iodinated prolactin. Ophthalmic Res 1982;14:54–62.
Arnold E, Thebault S, Baeza-Cruz G, et al. The hormone prolactin is a novel, endogenous trophic factor able to regulate reactive glia and to limit retinal degeneration. J Neurosci 2014;34:1868–78.
Kong L, Bhatt AR, Demny AB, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci 2015;56:956–61.
Pieh C, Agostini H, Buschbeck C, et al. VEGF-A, VEGFR-1, VEGFR-2 and Tie2 levels in plasma of premature infants: relationship to retinopathy of prematurity. Br J Ophthalmol 2008;92:689–93.
Woo SJ, Park KH, Lee SY, et al. The relationship between cord blood cytokine levels and perinatal factors and retinopathy of prematurity: a gestational age-matched case-control study. Invest Ophthalmol Vis Sci 2013;54:3434–9.
Acknowledgements
We thank Fernando López-Barrera, and Gabriel Nava, for their technical assistance, Fernanda Parada-Dávila, for statistic support, and Dorothy D. Pless, for critically editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zepeda-Romero, L., Vazquez-Membrillo, M., Adan-Castro, E. et al. Higher prolactin and vasoinhibin serum levels associated with incidence and progression of retinopathy of prematurity. Pediatr Res 81, 473–479 (2017). https://doi.org/10.1038/pr.2016.241
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.241
This article is cited by
-
Topical ophthalmic administration of the antiangiogenic peptide VIAN-c4551 protects against experimental diabetic macular edema
Scientific Reports (2025)
-
Levosulpiride for the treatment of diabetic macular oedema: a phase 2 randomized clinical trial
Eye (2024)
-
Deep Sc-RNA sequencing decoding the molecular dynamic architecture of the human retina
Science China Life Sciences (2023)
-
The HGR motif is the antiangiogenic determinant of vasoinhibin: implications for a therapeutic orally active oligopeptide
Angiogenesis (2022)
-
Vasoinhibin comprises a three-helix bundle and its antiangiogenic domain is located within the first 79 residues
Scientific Reports (2018)