Abstract
Background:
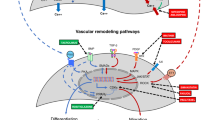
Bronchopulmonary dysplasia (BPD) is the most common and serious chronic lung disease of premature infants. Connective tissue growth factor (CTGF) plays an important role in tissue development and remodeling. We have previously shown that targeted overexpression of CTGF in alveolar type II epithelial cells results in BPD-like pathology and activates β-catenin in neonatal mice.
Methods:
Utilizing this transgenic mouse model and ICG001, a specific pharmacological inhibitor of β-catenin, we tested the hypothesis that β-catenin signaling mediates the effects of CTGF in the neonatal lung. Newborn CTGF mice and control littermates received ICG001 (10 mg/kg/dose) or placebo (dimethyl sulfoxide, equal volume) by daily i.p. injection from postnatal day 5 to 15. Alveolarization, vascular development, and pulmonary hypertension (PH) were analyzed.
Results:
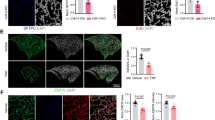
Administration of ICG001 significantly downregulated expression of cyclin D1, collagen 1a1, and fibronectin, which are the known target genes of β-catenin signaling in CTGF lungs. Inhibition of β-catenin signaling improved alveolar and vascular development and decreased pulmonary vascular remodeling. More importantly, the improved vascular development and vascular remodeling led to a decrease in PH.
Conclusion:
β-Catenin signaling mediates the autocrine and paracrine effects of CTGF in the neonatal lung. Inhibition of CTGF-β-catenin signaling may provide a novel therapy for BPD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9.
Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics 2009;123:1562–73.
Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 2005;67:623–61.
Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 2006;119:4803–10.
Kambas K, Chrysanthopoulou A, Kourtzelis I, et al. Endothelin-1 signaling promotes fibrosis in vitro in a bronchopulmonary dysplasia model by activating the extrinsic coagulation cascade. J Immunol 2011;186:6568–75.
Alapati D, Rong M, Chen S, et al. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol 2011;45:1169–77.
Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, et al. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2007;292:L537–49.
Chen CM, Wang LF, Chou HC, Lang YD, Lai YP. Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res 2007;62:128–33.
Shimada I, Matsui K, Brinkmann B, et al. Novel transcript profiling of diffuse alveolar damage induced by hyperoxia exposure in mice: normalization by glyceraldehyde 3-phosphate dehydrogenase. Int J Legal Med 2008;122:373–83.
Wu S, Capasso L, Lessa A, et al. High tidal volume ventilation up-regulates CTGF expression in the lung of newborn rats. Pediatr Res 2008;63:245–50.
Wu S, Platteau A, Chen S, McNamara G, Whitsett J, Bancalari E. Conditional overexpression of connective tissue growth factor disrupts postnatal lung development. Am J Respir Cell Mol Biol 2010;42:552–63.
Chen S, Rong M, Platteau A, et al. CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2011;300:L330–40.
Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin alpha(v)beta(3) and heparan sulfate proteoglycan. J Biol Chem 2004;279:8848–55.
Heng EC, Huang Y, Black SA Jr, Trackman PC. CCN2, connective tissue growth factor, stimulates collagen deposition by gingival fibroblasts via module 3 and alpha6- and beta1 integrins. J Cell Biochem 2006;98:409–20.
Mercurio S, Latinkic B, Itasaki N, Krumlauf R, Smith JC. Connective-tissue growth factor modulates WNT signalling and interacts with the WNT receptor complex. Development 2004;131:2137–47.
Deng YZ, Chen PP, Wang Y, et al. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem 2007;282:36571–81.
Emami KH, Nguyen C, Ma H, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA 2004;101:12682–7.
Alapati D, Rong M, Chen S, Hehre D, Hummler SC, Wu S. Inhibition of β-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2014;51:104–13.
Ivkovic S, Yoon BS, Popoff SN, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 2003;130:2779–91.
Sonnylal S, Shi-Wen X, Leoni P, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum 2010;62:1523–32.
Sonnylal S, Xu S, Jones H, et al. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J Cell Sci 2013;126:2164–75.
Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469–80.
Oloumi A, McPhee T, Dedhar S. Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochim Biophys Acta 2004;1691:1–15.
Dasgupta C, Sakurai R, Wang Y, et al. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol 2009;296:L1031–41.
Popova AP, Bentley JK, Anyanwu AC, et al. Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol 2012;303:L439–48.
Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 1999;96:5522–7.
Douglas IS, Diaz del Valle F, Winn RA, Voelkel NF. Beta-catenin in the fibroproliferative response to acute lung injury. Am J Respir Cell Mol Biol 2006;34:274–85.
Villar J, Cabrera NE, Valladares F, et al. Activation of the Wnt/β-catenin signaling pathway by mechanical ventilation is associated with ventilator-induced pulmonary fibrosis in healthy lungs. PLoS One 2011;6:e23914.
Baarsma HA, Spanjer AI, Haitsma G, et al. Activation of WNT/β-catenin signaling in pulmonary fibroblasts by TGF-β1 is increased in chronic obstructive pulmonary disease. PLoS One 2011;6:e25450.
Clarke DL, Carruthers AM, Mustelin T, Murray LA. Matrix regulation of idiopathic pulmonary fibrosis: the role of enzymes. Fibrogenesis Tissue Repair 2013;6:20.
Shafieian M, Chen S, Wu S. Integrin-linked kinase mediates CTGF-induced epithelial to mesenchymal transition in alveolar type II epithelial cells. Pediatr Res 2015;77:520–7.
Mucenski ML, Wert SE, Nation JM, et al. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–8.
Mucenski ML, Nation JM, Thitoff AR, et al. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 2005;289:L971–9.
Königshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol 2010;42:21–31.
Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest 2009;119:2538–49.
Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 2009;29:1074–9.
Wang X, LeMaire SA, Chen L, et al. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation 2006;114:Suppl 1:I200–5.
Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260–9.
Alapati D, Rong M, Chen S, Lin C, Li Y, Wu S. Inhibition of LRP5/6-mediated Wnt/β-catenin signaling by Mesd attenuates hyperoxia-induced pulmonary hypertension in neonatal rats. Pediatr Res 2013;73:719–25.
Duncan MR, Frazier KS, Abramson S, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J 1999;13:1774–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rong, M., Chen, S., Zambrano, R. et al. Inhibition of β-catenin signaling protects against CTGF-induced alveolar and vascular pathology in neonatal mouse lung. Pediatr Res 80, 136–144 (2016). https://doi.org/10.1038/pr.2016.52
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.52
This article is cited by
-
When inflammation meets lung development—an update on the pathogenesis of bronchopulmonary dysplasia
Molecular and Cellular Pediatrics (2022)
-
Modulation of the Notch System in Response to Wnt Inhibition Induces Restoration of the Rat Luteal Function
Reproductive Sciences (2020)