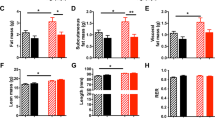
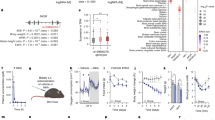
Abstract
Background:
Neonatal growth restriction (nGR) leads to leptin deficiency and increases the risk of hypertension. Previous studies have shown nGR-related hypertension is normalized by neonatal leptin (nLep) and exacerbated by psychological stress. With recent studies linking leptin and angiotensin signaling, we hypothesized that nGR-induced nLep deficiency increases adult leptin sensitivity; leading to leptin- or stress-induced hypertension, through a pathway involving central angiotensin II type 1 receptors.
Methods:
We randomized mice with incipient nGR, by virtue of their presence in large litters, to vehicle or physiologic nLep supplementation (80 ng/g/d). Adult caloric intake and arterial pressure were monitored at baseline, during intracerebroventricular losartan infusion and during systemic leptin administration.
Results:
nGR increased leptin-triggered renal sympathetic activation and hypertension with increased leptin receptor expression in the arcuate nucleus of the hypothalamus; all of those nGR-associated phenotypes were normalized by nLep. nGR mice also had stress-related hyperphagia and hypertension, but only the stress hypertension was blocked by central losartan infusion.
Conclusion:
nGR leads to stress hypertension through a pathway that involves central angiotensin II receptors, and nGR-associated leptin deficiency increases leptin-triggered hypertension in adulthood. These data suggest potential roles for preservation of neonatal growth and nLep supplementation in the prevention of nGR-related hypertension.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
World Health Organization. Low Birth Weight: A Tabulation of Available Information, WHO/MCH/92.2. World Health Organization, Geneva and UNICEF, New York, 1992.
Dagle JM, Fisher TJ, Haynes SE, et al. Cytochrome P450 (CYP2D6) genotype is associated with elevated systolic blood pressure in preterm infants after discharge from the neonatal intensive care unit. J Pediatr 2011;159:104–9.
Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol 2003;27:302–10.
Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 2005;112:3430–6.
Simonds SE, Pryor JT, Ravussin E, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell 2014;159:1404–16.
Briffa JF, McAinch AJ, Romano T, Wlodek ME, Hryciw DH. Leptin in pregnancy and development: a contributor to adulthood disease? Am J Physiol Endocrinol Metab 2015;308:E335–50.
Kawamata R, Suzuki Y, Yada Y, et al. Gut hormone profiles in preterm and term infants during the first 2 months of life. J Pediatr Endocrinol Metab 2014;27:717–23.
Spear ML, Hassink SG, Leef K, et al. Immaturity or starvation? Longitudinal study of leptin levels in premature infants. Biol Neonate 2001;80:35–40.
Sipola-Leppänen M, Vääräsmäki M, Tikanmäki M, et al. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol 2015;181:861–73.
Allison MA, Ix JH, Morgan C, et al. Higher leptin is associated with hypertension: the Multi-Ethnic Study of Atherosclerosis. J Hum Hypertens 2013;27:617–22.
Dubinion JH, do Carmo JM, Adi A, Hamza S, da Silva AA, Hall JE. Role of proopiomelanocortin neuron Stat3 in regulating arterial pressure and mediating the chronic effects of leptin. Hypertension 2013;61:1066–74.
Hilzendeger AM, Morgan DA, Brooks L, et al. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol 2012;303:H197–206.
Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS. Development of metabolic systems. Physiol Behav 2005;86:646–60.
Erkonen GE, Hermann GM, Miller RL, et al. Neonatal leptin administration alters regional brain volumes and blocks neonatal growth restriction-induced behavioral and cardiovascular dysfunction in male mice. Pediatr Res 2011;69(5 Pt 1):406–12.
Hermann GM, Miller RL, Erkonen GE, et al. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatr Res 2009;66:53–8.
Meyer LR, Zhu V, Miller A, Roghair RD. Growth restriction, leptin, and the programming of adult behavior in mice. Behav Brain Res 2014;275:131–5.
Johansson S, Norman M, Legnevall L, Dalmaz Y, Lagercrantz H, Vanpée M. Increased catecholamines and heart rate in children with low birth weight: perinatal contributions to sympathoadrenal overactivity. J Intern Med 2007;261:480–7.
Morgan DA, Thedens DR, Weiss R, Rahmouni K. Mechanisms mediating renal sympathetic activation to leptin in obesity. Am J Physiol Regul Integr Comp Physiol 2008;295:R1730–6.
Eriksson JG, Forsén TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension 2007;49:1415–21.
Pladys P, Sennlaub F, Brault S, et al. Microvascular rarefaction and decreased angiogenesis in rats with fetal programming of hypertension associated with exposure to a low-protein diet in utero. Am J Physiol Regul Integr Comp Physiol 2005;289:R1580–8.
Dodic M, McAlinden AT, Jefferies AJ, et al. Differential effects of prenatal exposure to dexamethasone or cortisol on circulatory control mechanisms mediated by angiotensin II in the central nervous system of adult sheep. J Physiol 2006;571(Pt 3):651–60.
Roghair RD, Aldape G. Naturally occurring perinatal growth restriction in mice programs cardiovascular and endocrine function in a sex- and strain-dependent manner. Pediatr Res 2007;62:399–404.
Pyhälä R, Räikkönen K, Feldt K, et al. Blood pressure responses to psychosocial stress in young adults with very low birth weight: Helsinki study of very low birth weight adults. Pediatrics 2009;123:731–4.
Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol 2004;287:R1394–8.
Crume TL, Scherzinger A, Stamm E, et al. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring) 2014;22:608–15.
Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics 2003;112(1 Pt 1):e30–8.
Porter JP, Potratz KR. Effect of intracerebroventricular angiotensin II on body weight and food intake in adult rats. Am J Physiol Regul Integr Comp Physiol 2004;287:R422–8.
Jones A, Beda A, Ward AM, et al. Size at birth and autonomic function during psychological stress. Hypertension 2007;49:548–55.
Kuo JJ, Jones OB, Hall JE. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension 2001;37(2 Pt 2):670–6.
Roghair RD, Segar JL, Volk KA, et al. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. Am J Physiol Regul Integr Comp Physiol 2009;296:R651–62.
Harlan SM, Morgan DA, Agassandian K, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 2011;108:808–12.
Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004;145:4880–9.
Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol 2013;304:H1538–46.
Pighetti M, Tommaselli GA, D’Elia A, et al. Maternal serum and umbilical cord blood leptin concentrations with fetal growth restriction. Obstet Gynecol 2003;102:535–43.
Su PH, Wang SL, Chen JY, Lai CP, Jian SH. Serum leptin levels in preterm, healthy and sick-term newborns. Acta Paediatr Taiwan 2002;43:249–54.
Attig L, Djiane J, Gertler A, et al. Study of hypothalamic leptin receptor expression in low-birth-weight piglets and effects of leptin supplementation on neonatal growth and development. Am J Physiol Endocrinol Metab 2008;295:E1117–25.
Matochik JA, London ED, Yildiz BO, et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab 2005;90:2851–4.
WHO 1995 Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 854:1–452.
Roghair RD, Wemmie JA, Volk KA, Scholz TD, Lamb FS, Segar JL. Maternal antioxidant blocks programmed cardiovascular and behavioural stress responses in adult mice. Clin Sci (Lond) 2011;121:427–36.
Grobe JL, Grobe CL, Beltz TG, et al. The brain Renin-angiotensin system controls divergent efferent mechanisms to regulate fluid and energy balance. Cell Metab 2010;12:431–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peotta, V., Rahmouni, K., Segar, J. et al. Neonatal growth restriction-related leptin deficiency enhances leptin-triggered sympathetic activation and central angiotensin II receptor-dependent stress-evoked hypertension. Pediatr Res 80, 244–251 (2016). https://doi.org/10.1038/pr.2016.64
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.64