Abstract
Background:
Perinatal hypoxic–ischemic brain damage is a major cause of mortality and morbidity in the neonatal period. Currently, limited ranges of biochemical tests assessing the intensity and duration of hypoxia are ready for clinical use. However, the need to initiate hypothermia therapy early after the clinical suspicion of hypoxic–ischemic encephalopathy requires the availability of early and reliable hypoxia markers. We have sought these biomarkers in an experimental model of hypoxia reoxygenation.
Methods:
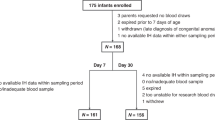
Hypoxia and hypotension were induced in newborn piglets following a standardized model and reoxygenation was carried out using room air (RA). An untargeted liquid chromatography—time of flight mass spectrometry (LC-TOFMS) approach was used to assess changes in the metabolomic profile of plasma samples after intense hypoxia and upon reoxygenation.
Results:
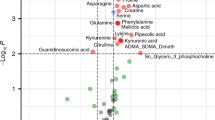
At the end of hypoxia, the plasma metabolome showed an increased plasma concentration of analytes reflecting a metabolic adaptation to prolonged anaerobiosis. However, after resuscitation, metabolite levels returned to the starting values.
Conclusion:
Severe hypoxia induces early, significant, and transient changes of specific metabolites in the plasma metabolome, which represent a snapshot of the biochemical adaptation of mammals to intense hypoxia. These metabolites could have applicability in predicting the severity of hypoxia in the clinical setting.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gunn AJ, Bennet L. Fetal hypoxia insults and patterns of brain injury: insights from animal models. Clin Perinatol 2009;36:579–93.
Grivell RM, Alfirevic Z, Gyte GM, Devane D. Antenatal cardiotocography for fetal assessment. Cochrane Database Syst Rev 2012;12:CD007863.
Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG 2012;119:824–31.
Shankaran S, Pappas A, McDonald SA, et al.; Eunice Kennedy Shriver NICHD Neonatal Research Network. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012;366:2085–92.
Committee on Fetus and Newborn, Papile L-A, Baley JE, et al. Hypothermia and neonatal encephalopathy. Pediatrics 2014;133:1146–50.
Solberg R, Enot D, Deigner HP, et al. Metabolomic analyses of plasma reveals new insights into asphyxia and resuscitation in pigs. PLoS One 2010;5:e9606.
Yin P, Xu G. Current state-of-the-art of nontargeted metabolomics based on liquid chromatography-mass spectrometry with special emphasis in clinical applications. J Chromatogr A 2014;1374:1–13.
Skappak C, Regush S, Cheung PY, Adamko DJ. Identifying hypoxia in a newborn piglet model using urinary NMR metabolomic profiling. PLoS One 2013;8:e65035.
Murgia F, Noto A, Iacovidou N, et al. Is the quickness of resuscitation after hypoxia influenced by the oxygen concentration? Metabolomics in piglets resuscitated with different oxygen concentrations. J Pediatr Neonatal Individ Med JPNIM 2013;2:e020233.
Solberg R, Escobar J, Arduini A, et al. Metabolomic analysis of the effect of postnatal hypoxia on the retina in a newly born Piglet model. PLoS One 2013;8:e66540.
Fiedorowicz M, Makarewicz D, Stańczak-Mrozek KI, Grieb P. CDP-choline (citicoline) attenuates brain damage in a rat model of birth asphyxia. Acta Neurobiol Exp (Wars) 2008;68:389–97.
Andresen JH, Carlsen B, Solberg R, et al. Newborn piglets exposed to hypoxia after nicotine or saline pretreatment: long-term effects on brain and heart. J Matern Fetal Neonatal Med 2009;22:161–8.
Gromski PS, Xu Y, Correa E, Ellis DI, Turner ML, Goodacre R. A comparative investigation of modern feature selection and classification approaches for the analysis of mass spectrometry data. Anal Chim Acta 2014;829:1–8.
Xia J, Wishart DS. MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res 2010;38(Web Server issue):W71–7.
Lourenço AB, Roque FC, Teixeira MC, Ascenso JR, Sá-Correia I. Quantitative 1H-NMR-metabolomics reveals extensive metabolic reprogramming and the effect of the aquaglyceroporin FPS1 in ethanol-stressed yeast cells. PLoS One 2013;8:e55439.
Gibellini F, Smith TK. The Kennedy pathway-de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUMBM Life 2012;2:414–28.
Saugstad OD. Hypoxanthine as a measurement of hypoxia. Pediatr Res 1975;9:158–61.
Saugstad OD. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr Res 1988;23:143–50.
Rauchová H, Vokurková M, Koudelová J. Hypoxia-induced lipid peroxidation in the brain during postnatal ontogenesis. Physiol Res 2012;61 Suppl 1:S89–101.
Dehne N, Brüne B. Sensors, transmitters, and targets in mitochondrial oxygen shortage-a hypoxia-inducible factor relay story. Antioxid Redox Signal 2014;20:339–52.
Muratsubaki H, Yamaki A. Profile of plasma amino acid levels in rats exposed to acute hypoxic hypoxia. Indian J Clin Biochem 2011;26:416–9.
Chiang JY. Bile acid metabolism and signaling. Compr Physiol 2013;3:1191–212.
Kelly A, Stanley CA. Disorders of glutamate metabolism. Ment Retard Dev Disabil Res Rev 2001;7:287–95.
Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem Funct 2003;21:1–9.
Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0–a comprehensive server for metabolomic data analysis. Nucleic Acids Res 2012;40(Web Server issue):W127–33.
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50.
Nam D, Kim SY. Gene-set approach for expression pattern analysis. Brief Bioinform 2008;9:189–97.
Goeman JJ, van de Geer SA, de Kort F, van Houwelingen HC. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics 2004;20:93–9.
Wang SY, Kuo CH, Tseng YJ. Batch Normalizer: a fast total abundance regression calibration method to simultaneously adjust batch and injection order effects in liquid chromatography/time-of-flight mass spectrometry-based metabolomics data and comparison with current calibration methods. Anal Chem 2013;85:1037–46.
Acknowledgements
We would like to thank the Servicio de Soporte a la Investigación Experimental (SCSIE) of the University of Valencia (Spain).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Material
(ZIP 109 kb)
Rights and permissions
About this article
Cite this article
Solberg, R., Kuligowski, J., Pankratov, L. et al. Changes of the plasma metabolome of newly born piglets subjected to postnatal hypoxia and resuscitation with air. Pediatr Res 80, 284–292 (2016). https://doi.org/10.1038/pr.2016.66
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.66
This article is cited by
-
Circulating choline levels are associated with prognoses in patients with pulmonary hypertension: a cohort study
BMC Pulmonary Medicine (2023)
-
Metabolomics improves the histopathological diagnosis of asphyxial deaths: an animal proof-of-concept model
Scientific Reports (2021)
-
Metabolic adaptations to hypoxia in the neonatal mouse forebrain can occur independently of the transporters SLC7A5 and SLC3A2
Scientific Reports (2021)
-
Oxygen therapy of the newborn from molecular understanding to clinical practice
Pediatric Research (2019)
-
Assessment of phospholipid synthesis related biomarkers for perinatal asphyxia: a piglet study
Scientific Reports (2017)