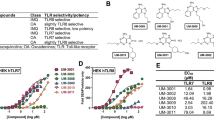
Abstract
Background:
Preterm neonates display an impaired vaccine response. Neonatal antigen-presenting cells (APCs) are less effective to induce an adaptive immune response and to promote the development of immunological memory. Efficient adjuvantal toll-like receptor (TLR)-triggering may overcome the neonatal immunological impairment. Accordingly, the aim of this study was to investigate the immunostimulatory action of R-848 and CpG-B on neonatal APCs.
Methods:
Surface marker and cytokine secretion of APCs were evaluated after incubation of cord blood and peripheral blood mononuclear cells with the indicated adjuvants and were analyzed using flow cytometry.
Results:
TLR-specific stimulation resulted in a significant induction of costimulatory molecules on neonatal APCs. Stimulation with R-848 resulted in significant higher secretion of TNFα, IL-6, IL-10, IL-12/IL-23p40, IL-12p70, and IFN-γ. Interestingly, CpG-B resulted in significant higher secretion of TNFα and IL-6.
Conclusion:
In summary, the incubation of TLR-agonists induced activation and maturation of neonatal APCs. These data show that modern TLR-specific adjuvants achieve a direct effect and potent upregulation of activation and maturation markers and cytokines in preterm neonates. We thus conclude that agents triggering TLRs might possibly overcome neonatal lack of vaccine responses.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bonhoeffer J, Siegrist CA, Heath PT. Immunisation of premature infants. Arch Dis Child 2006;91:929–35.
Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285–91.
D’Angio CT, Heyne RJ, Duara S, et al.; Premature Infant Vaccine Collaborative. Immunogenicity of trivalent influenza vaccine in extremely low-birth-weight, premature versus term infants. Pediatr Infect Dis J 2011;30:570–4.
Shinefield H, Black S, Ray P, Fireman B, Schwalbe J, Lewis E. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr Infect Dis J 2002;21:182–6.
Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007;7:379–90.
Wynn JL, Levy O. Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin Perinatol 2010;37:307–37.
Förster-Waldl E, Sadeghi K, Tamandl D, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res 2005;58:121–4.
Leja M, Cine E, Rudzite D, et al. Prevalence of Helicobacter pylori infection and atrophic gastritis in Latvia. Eur J Gastroenterol Hepatol 2012;24:1410–7.
Garcia AM, Fadel SA, Cao S, Sarzotti M. T cell immunity in neonates. Immunol Res 2000;22:177–90.
Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine 2011;29:3341–55.
Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS 2005;19:1473–9.
Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol 2004;24:693–701.
Mullen GE, Ellis RD, Miura K, et al. Phase 1 trial of AMA1-C1/Alhydrogel plus CPG 7909: an asexual blood-stage vaccine for Plasmodium falciparum malaria. PLoS One 2008;3:e2940.
Boraschi D, Aguado MT, Dutel C, et al. The gracefully aging immune system. Sci Transl Med 2013;5:185ps8.
Siegrist CA. Neonatal and early life vaccinology. Vaccine 2001;19:3331–46.
Siegrist CA. Vaccination in the neonatal period and early infancy. Int Rev Immunol 2000;19:195–219.
Schüller SS, Sadeghi K, Wisgrill L, et al. Preterm neonates display altered plasmacytoid dendritic cell function and morphology. J Leukoc Biol 2013;93:781–8.
Lavoie PM, Huang Q, Jolette E, et al. Profound lack of interleukin (IL)-12/IL-23p40 in neonates born early in gestation is associated with an increased risk of sepsis. J Infect Dis 2010;202:1754–63.
Katashiba Y, Miyamoto R, Hyo A, et al. Interferon-α and interleukin-12 are induced, respectively, by double-stranded DNA and single-stranded RNA in human myeloid dendritic cells. Immunology 2011;132:165–73.
Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 2006;108:1284–90.
Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009;27:3331–4.
Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol 2012;61(Pt 7):927–34.
Sharma AA, Jen R, Brant R, et al. Hierarchical maturation of innate immune defences in very preterm neonates. Neonatology 2014;106:1–9.
Philbin VJ, Dowling DJ, Gallington LC, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 2012;130:195–204.e9.
Wille-Reece U, Flynn BJ, Loré K, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA 2005;102:15190–4.
Wille-Reece U, Flynn BJ, Loré K, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med 2006;203:1249–58.
Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol 2005;174:7676–83.
Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res 2012;18:6748–57.
Feyerabend S, Stevanovic S, Gouttefangeas C, et al. Novel multi-peptide vaccination in Hla-A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate 2009;69:917–27.
Sagara I, Ellis RD, Dicko A, et al. A randomized and controlled Phase 1 study of the safety and immunogenicity of the AMA1-C1/Alhydrogel + CPG 7909 vaccine for Plasmodium falciparum malaria in semi-immune Malian adults. Vaccine 2009;27:7292–8.
Cooper CL, Davis HL, Morris ML, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine 2004;22:3136–43.
Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis 2008;46:1310–4.
Siegrist CA, Pihlgren M, Tougne C, et al. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine 2004;23:615–22.
Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 1997;185:1101–11.
Acknowledgements
We would like to thank Günther Hofbauer, Core Unit for Flow Cytometry, Medical University of Vienna, for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schüller, S., Wisgrill, L., Sadeghi, K. et al. The TLR-specific adjuvants R-848 and CpG-B endorse the immunological reaction of neonatal antigen-presenting cells. Pediatr Res 80, 311–318 (2016). https://doi.org/10.1038/pr.2016.71
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.71
This article is cited by
-
Modified Fe3O4 Magnetic Nanoparticle Delivery of CpG Inhibits Tumor Growth and Spontaneous Pulmonary Metastases to Enhance Immunotherapy
Nanoscale Research Letters (2018)
-
Endothelial cells of extremely premature infants display impaired immune response after proinflammatory stimulation
Pediatric Research (2018)