Abstract
Background:
Preterm infants are vulnerable to pathogens and at risk of developing necrotizing enterocolitis (NEC) or sepsis. Nasogastric feeding tubes (NG-tubes) might contaminate feeds given through them due to biofilm formation. We wanted to determine if there is a rationale in replacing NG-tubes more often to reduce contamination.
Methods:
We conducted an observational study of used NG-tubes from a tertiary neonatal department. After removal, we flushed a 1-ml saline solution through the tube, determined the density of bacteria by culture, and related it to the duration of use and any probiotic administration through the tube.
Results:
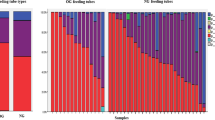
Out of the 94 NG-tubes, 89% yielded more than 1,000 colony-forming units (CFU)/ml bacteria, and 55% yielded the potentially pathogenic Enterobacteriaceae and/or Staphylococcus aureus. The mean concentration in the yield was 5.3 (SD: 2.1, maximum 9.4) log10CFU/ml. Neither the presence of contamination nor the density was associated with the time the NG-tube had been in use. Probiotic administration did not protect against contamination.
Conclusion:
NG-tubes yielded high densities of bacteria even within the first day of use. Further studies are needed to determine if changing the NG-tubes between meals or once a day will make a positive impact on tube contamination and clinical parameters.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hackam DJ, Good M, Sodhi CP. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin Pediatr Surg 2013;22:76–82.
Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr 1999;23(5 Suppl):S3–6.
Veereman-Wauters G. Neonatal gut development and postnatal adaptation. Eur J Pediatr 1996;155:627–32.
Brooks B, Firek BA, Miller CS, et al. Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2014;2:1.
Collado MC, Cernada M, Neu J, Pérez-Martínez G, Gormaz M, Vento M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res 2015;77:726–31.
Hurrell E, Kucerova E, Loughlin M, et al. Neonatal enteral feeding tubes as loci for colonisation by members of the Enterobacteriaceae. BMC Infect Dis 2009;9:146.
Mehall JR, Kite CA, Saltzman DA, Wallett T, Jackson RJ, Smith SD. Prospective study of the incidence and complications of bacterial contamination of enteral feeding in neonates. J Pediatr Surg 2002;37:1177–82.
Patel AL, Trivedi S, Bhandari NP, et al. Reducing necrotizing enterocolitis in very low birth weight infants using quality-improvement methods. J Perinatol 2014;34:850–7.
Hein-Nielsen AL, Petersen SM, Greisen G. Unchanged incidence of necrotising enterocolitis in a tertiary neonatal department. Dan Med J 2015;62:.
Kristoffersen L, Skogvoll E, Hafström M. Pain reduction on insertion of a feeding tube in preterm infants: a randomized controlled trial. Pediatrics 2011;127:e1449–54.
Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2011;111:CD005496.
McMillan A, Dell M, Zellar MP, et al. Disruption of urogenital biofilms by lactobacilli. Colloids Surf B Biointerfaces 2011;86:58–64.
Hurrell E, Kucerova E, Loughlin M, Caubilla-Barron J, Forsythe SJ. Biofilm formation on enteral feeding tubes by Cronobacter sakazakii, Salmonella serovars and other Enterobacteriaceae. Int J Food Microbiol 2009;136:227–31.
Patel K, Konduru K, Patra AK, Chandel DS, Panigrahi P. Trends and determinants of gastric bacterial colonization of preterm neonates in a NICU setting. PLoS One 2015;10:e0114664.
Milisavljevic V, Garg M, Vuletic I, et al. Prospective assessment of the gastroesophageal microbiome in VLBW neonates. BMC Pediatr 2013;13:49.
Hoy CM, Wood CM, Hawkey PM, Puntis JW. Duodenal microflora in very-low-birth-weight neonates and relation to necrotizing enterocolitis. J Clin Microbiol 2000;38:4539–47.
Li YF, Lin HC, Torrazza RM, Parker L, Talaga E, Neu J. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatr Neonatol 2014;55:335–40.
Aly H, Badawy M, Tomerak RH, El-Kholy AA, Hamed AS. Tracheal colonization in preterm infants supported with nasal continuous positive airway pressure. Pediatr Int 2012;54:356–60.
Parm Ü, Metsvaht T, Ilmoja ML, Lutsar I. Gut colonization by aerobic microorganisms is associated with route and type of nutrition in premature neonates. Nutr Res 2015;35:496–503.
Hickey L, Garland SM, Jacobs SE, O’Donnell CP, Tabrizi SN ; ProPrems Study Group. Cross-colonization of infants with probiotic organisms in a neonatal unit. J Hosp Infect 2014;88:226–9.
Neu J. Probiotics and necrotizing enterocolitis. Clin Perinatol 2014;41:967–78.
DANMAP 2014 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600–2032. (http://www.danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202014/Danmap_2014.ashx)
Lynch JP 3rd, Clark NM, Zhanel GG. Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum β-lactamases and carbapenemases). Expert Opin Pharmacother 2013;14:199–210.
Jones V, Wilks M, Johnson G, et al. The use of molecular techniques for bacterial detection in the analysis of gastric aspirates collected from infants on the first day of life. Early Hum Dev 2010;86:167–70.
Berthelot P, Grattard F, Patural H, et al. Nosocomial colonization of premature babies with Klebsiella oxytoca: probable role of enteral feeding procedure in transmission and control of the outbreak with the use of gloves. Infect Control Hosp Epidemiol 2001;22:148–51.
Yong SB, Ma JS, Chen FS, Chung MY, Yang KD. Nasogastric tube placement and esophageal perforation in extremely low birth weight infants. Pediatr Neonatol (2013), http://dx.doi.org/10.1016/j.pedneo.2013.10.011.
Stokkou S, Geginat G, Schlüter D, Tammer I. Direct disk diffusion test using European Clinical Antimicrobial Susceptibility Testing breakpoints provides reliable results compared with the standard method. Eur J Microbiol Immunol (Bp) 2015;5:103–11.
Acknowledgements
Thanks to the nurses at the neonatal department for collecting the nasogastric tubes, and to Susanne Schjørring, Michala Sørensen, Rie Jønsson, Christina Tingbjerg Brandt, Steffen Jørgensen, and Maj Holm for their contributions in the laboratory at Statens Serum Institute. We acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Petersen, S., Greisen, G. & Krogfelt, K. Nasogastric feeding tubes from a neonatal department yield high concentrations of potentially pathogenic bacteria— even 1 d after insertion. Pediatr Res 80, 395–400 (2016). https://doi.org/10.1038/pr.2016.86
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.86
This article is cited by
-
A comparison of bacterial colonization between nasogastric and orogastric enteral feeding tubes in infants in the neonatal intensive care unit
Journal of Perinatology (2022)
-
Nasogastric enteral feeding tubes modulate preterm colonization in early life
Pediatric Research (2022)
-
Transitioning from gavage to full oral feeds in premature infants: When should we discontinue the nasogastric tube?
Journal of Perinatology (2019)
-
Microbiological monitoring of continuous positive airway pressure and resuscitation equipment in very-low birth weight infants
Pediatric Research (2018)
-
Rapid in situ imaging and whole genome sequencing of biofilm in neonatal feeding tubes: A clinical proof of concept
Scientific Reports (2017)