Abstract
Background:
Kawasaki disease (KD) is the most common systemic vasculitis syndrome primarily affecting medium-sized arteries, particularly the coronary arteries. Though KD may be associated with immunological problems, the involvement of microRNAs (miRs) has not been fully described.
Methods:
We enrolled 23 KD patients and 12 controls. We performed miR and mRNA microarray analysis of peripheral blood mononuclear cells (PBMCs) isolated from acute KD patients and controls. Continuously, we measured specific miRs, mRNA and the expression of proteins by using reverse-transcriptase PCR (RT-PCR) and enzyme-linked immunosorbent assay (ELISA).
Results:
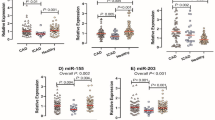
We identified strikingly high levels of miR-182 and miR-296-5p during the acute febrile phase, and of miR-93, miR-145-5p, miR-145-3p, and miR-150-3p in the defervescence stage, especially in refractory KD patients. The expression of vascular endothelial growth factor A (VEGFA) mRNA, previously reported to be controlled by miR-93, was significantly elevated during the febrile phase and normalized upon treatment, negatively correlating with the expression of miR-93. Further, plasma levels of VEGF-A correlated with PBMC VEGFA mRNA expression.
Conclusion:
Several miRs are highly specific to the acute phase of KD, and may participate in regulating the expression of genes in pathways associated with KD. In particular, miR-93 may participate in regulating expression of VEGF-A and contribute to the pathogenesis of arteritis in acute KD.
Similar content being viewed by others

Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatr 1974;54:271–6.
Terai M, Yasukawa K, Narumoto S, Tateno S, Oana S, Kohno Y. Vascular endothelial growth factor in acute Kawasaki disease. Am J Cardiol 1999;83:337–9.
Hamamichi Y, Ichida F, Yu X, et al. Neutrophils and mononuclear cells express vascular endothelial growth factor in acute Kawasaki disease: its possible role in progression of coronary artery lesions. Pediatr Res 2001;49:74–80.
Greco A, De Virgilio A, Rizzo MI, et al. Kawasaki disease: an evolving paradigm. Autoimmun Rev 2015;14:703–9.
Wang Y, Wang W, Gong F, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum 2013;65:805–14.
Jia S, Li C, Wang G, Yang J, Zu Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin Exp Immunol 2010;162:131–7.
Crome SQ, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol 2010;159:109–19.
Afzali B, Mitchell P, Lechler RI, John S, Lombardi G. Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells. Clin Exp Immunol 2010;159:120–30.
Ichiyama T, Yoshitomi T, Nishikawa M, et al. NF-kappaB activation in peripheral blood monocytes/macrophages and T cells during acute Kawasaki disease. Clin Immunol 2001;99:373–7.
Cursiefen C, Chen L, Borges LP, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004;113:1040–50.
Kroll J, Waltenberger J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem Biophys Res Commun 1998;252:743–6.
Kaneda R, Fukuda K. MicroRNA is a new diagnostic and therapeutic target for cardiovascular disease and regenerative medicine. Circ J 2009;73:1397–8.
Kawashima T, Shioi T. MicroRNA, emerging role as a biomarker of heart failure. Circ J 2011;75:268–9.
De Rosa S, Curcio A, Indolfi C. Emerging role of microRNAs in cardiovascular diseases. Circ J 2014;78:567–75.
Sonkoly E, Ståhle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol 2008;18:131–40.
Ichiyama T, Ueno Y, Hasegawa M, Niimi A, Matsubara T, Furukawa S. Intravenous immunoglobulin inhibits NF-kappaB activation and affects Fcgamma receptor expression in monocytes/macrophages. Naunyn Schmiedebergs Arch Pharmacol 2004;369:428–33.
Shimizu C, Kim J, Stepanowsky P, et al. Differential expression of miR-145 in children with Kawasaki disease. PLoS One 2013;8:e58159.
Abe J, Jibiki T, Noma S, Nakajima T, Saito H, Terai M. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J Immunol 2005;174:5837–45.
Stacker SA, Vitali A, Caesar C, et al. A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J Biol Chem 1999;274:34884–92.
Larrivée B, Karsan A. Signaling pathways induced by vascular endothelial growth factor (review). Int J Mol Med 2000;5:447–56.
Shimizu C, Jain S, Davila S, et al. Transforming growth factor-beta signaling pathway in patients with Kawasaki disease. Circ Cardiovasc Genet 2011;4:16–25.
Yu X, Hirono KI, Ichida F, et al. Enhanced iNOS expression in leukocytes and circulating endothelial cells is associated with the progression of coronary artery lesions in acute Kawasaki disease. Pediatr Res 2004;55:688–94.
Foell D, Ichida F, Vogl T, et al. S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet 2003;361:1270–2.
Hirono K, Foell D, Xing Y, et al. Expression of myeloid-related protein-8 and -14 in patients with acute Kawasaki disease. J Am Coll Cardiol 2006;48:1257–64.
Burns JC. S100 proteins in the pathogenesis of Kawasaki disease. J Am Coll Cardiol 2006;48:1265–7.
Wittkowski H, Hirono K, Ichida F, et al. Acute Kawasaki disease is associated with reverse regulation of soluble receptor for advance glycation end products and its proinflammatory ligand S100A12. Arthritis Rheum 2007;56:4174–81.
Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007;81:28–37.
Ye F, Foell D, Hirono KI, et al. Neutrophil-derived S100A12 is profoundly upregulated in the early stage of acute Kawasaki disease. Am J Cardiol 2004;94:840–4.
Viemann D, Strey A, Janning A, et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 2005;105:2955–62.
Kelada S, Sethupathy P, Okoye IS, et al. miR-182 and miR-10a are key regulators of Treg specialisation and stability during Schistosome and Leishmania-associated inflammation. PLoS Pathog 2013;9:e1003451.
Ni FF, Li CR, Li Q, Xia Y, Wang GB, Yang J. Regulatory T cell microRNA expression changes in children with acute Kawasaki disease. Clin Exp Immunol 2014;178:384–93.
Anand S, Cheresh DA. Emerging role of Micro-RNAs in the regulation of angiogenesis. Genes Cancer 2011;2:1134–8.
Kawasaki T. Clinical signs and symptoms of mucocutaneous lymph node syndrome (Kawasaki disease). Jpn J Med Sci Biol 1979;32:237–8.
JCS Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Digest version. Circ J 2014;78:2521–62.
Takasaki I, Takarada S, Fukuchi M, Yasuda M, Tsuda M, Tabuchi Y. Identification of genetic networks involved in the cell growth arrest and differentiation of a rat astrocyte cell line RCG-12. J Cell Biochem 2007;102:1472–85.
Kiriakidou M, Nelson PT, Kouranov A, et al. A combined computational-experimental approach predicts human microRNA targets. Genes Dev 2004;18:1165–78.
Megraw M, Sethupathy P, Corda B, Hatzigeorgiou AG. miRGen: a database for the study of animal microRNA genomic organization and function. Nucleic Acids Res 2007;35(Database issue):D149–55.
Sethupathy P, Corda B, Hatzigeorgiou AG. TarBase: A comprehensive database of experimentally supported animal microRNA targets. RNA 2006;12:192–7.
Acknowledgements
We thank our colleagues and collaborating hospitals for always supporting us: Hitoshi Moriuchi, Chikako Sakai, Ikuo Hashimoto, Kentaro Tamura, Kiyoshi Hatasaki, Kentaro Shinozaki, Yasunori Ito, Hisano Nakatsubo, Satoshi Umekawa, Syunji Ishihara, Tatsuya Fuchizawa, Junko Yamamoto, Keiichiro Uese, Osamu Higuchi, Motokazu Nakabayashi, Takashi Kuramoto, Shinichi Tsubata, Seiko Saito, Taketoshi Yoshida, Hirokazu Kanegane, Keisuke Ohtsubo, Toshiko Itazawa, and Yuichi Adachi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, K., Nakaoka, H., Takasaki, I. et al. MicroRNA-93 may control vascular endothelial growth factor A in circulating peripheral blood mononuclear cells in acute Kawasaki disease. Pediatr Res 80, 425–432 (2016). https://doi.org/10.1038/pr.2016.93
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2016.93
This article is cited by
-
Genome-wide transcriptome analysis to further understand neutrophil activation and lncRNA transcript profiles in Kawasaki disease
Scientific Reports (2019)
-
MicroRNA-145-5p and microRNA-320a encapsulated in endothelial microparticles contribute to the progression of vasculitis in acute Kawasaki Disease
Scientific Reports (2018)