Abstract
Background:
High-dose erythropoietin (Epo) is a promising neuroprotective treatment in neonates with hypoxic–ischemic encephalopathy (HIE) receiving hypothermia. We evaluated the pharmacokinetics and dose–exposure relationships of high-dose Epo in this population to inform future dosing strategies.
Methods:
We performed a population pharmacokinetic analysis of 47 neonates with HIE treated with hypothermia who received up to six doses of Epo in two previous clinical trials. We compared the ability of different dosing regimens to achieve the target neuroprotective Epo exposure levels determined from animal models of hypoxic–ischemia (i.e., area under the curve during the first 48 h of treatment (AUC48 h) 140,000 mU*h/ml).
Results:
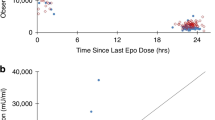
Birth weight scaled via allometry was a significant predictor of Epo clearance and volume of distribution (P < 0.001). After accounting for birth weight, variation in Epo pharmacokinetics between neonates was low (CV% 20%). All 23 neonates who received 1,000 U/kg every 24 h for the first 2 d of therapy achieved the target AUC48 h 140,000 mU*h/ml. No neonate who received a lower dosing regimen achieved this target.
Conclusion:
In neonates with HIE receiving hypothermia, Epo 1,000 U/kg every 24 h for the first 2 d of therapy resulted in consistent achievement of target exposures associated with neuroprotection in animal models.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Perlman JM, Wyllie J, Kattwinkel J, et al.; Neonatal Resuscitation Chapter Collaborators. Part 11: Neonatal resuscitation: 2010 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2010;122(16 Suppl 2):S516–38.
Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70.
Jacobs SE, Morley CJ, Inder TE, et al.; Infant Cooling Evaluation Collaboration. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med 2011;165:692–700.
Simbruner G, Mittal RA, Rohlmann F, Muche R ; neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 2010;126:e771–8.
Azzopardi DV, Strohm B, Edwards AD, et al.; TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58.
Shankaran S, Pappas A, McDonald SA, et al.; Eunice Kennedy Shriver NICHD Neonatal Research Network. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012;366:2085–92.
Kumral A, Ozer E, Yilmaz O, et al. Neuroprotective effect of erythropoietin on hypoxic-ischemic brain injury in neonatal rats. Biol Neonate 2003;83:224–8.
Kumral A, Uysal N, Tugyan K, et al. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav Brain Res 2004;153:77–86.
Matsushita H, Johnston MV, Lange MS, Wilson MA. Protective effect of erythropoietin in neonatal hypoxic ischemia in mice. Neuroreport 2003;14:1757–61.
Demers EJ, McPherson RJ, Juul SE. Erythropoietin protects dopaminergic neurons and improves neurobehavioral outcomes in juvenile rats after neonatal hypoxia-ischemia. Pediatr Res 2005;58:297–301.
Spandou E, Papadopoulou Z, Soubasi V, et al. Erythropoietin prevents long-term sensorimotor deficits and brain injury following neonatal hypoxia-ischemia in rats. Brain Res 2005;1045:22–30.
Sun Y, Calvert JW, Zhang JH. Neonatal hypoxia/ischemia is associated with decreased inflammatory mediators after erythropoietin administration. Stroke 2005;36:1672–8.
Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in brain-injured neonatal rats. Pediatr Res 2007;61:451–5.
Kumral A, Tüzün F, Oner MG, Genç S, Duman N, Ozkan H. Erythropoietin in neonatal brain protection: the past, the present and the future. Brain Dev 2011;33:632–43.
Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics 2009;124:e218–26.
Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics 2010;125:e1135–42.
Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics 2016;137:e20160191.
Frymoyer A, Meng L, Bonifacio SL, Verotta D, Guglielmo BJ. Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy 2013;33:718–26.
Frymoyer A, Lee S, Bonifacio SL, et al. Every 36-h gentamicin dosing in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. J Perinatol 2013;33:778–82.
Frymoyer A, Bonifacio SL, Drover DR, Su F, Wustoff CJ, Van Meurs KP. Decreased morphine clearance in neonates with hypoxic ischemic encephalopathy receiving hypothermia. J Clin Pharmacol 2017;57:64–76.
Statler PA, McPherson RJ, Bauer LA, Kellert BA, Juul SE. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res 2007;61:671–5.
Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics 2012;130:683–91.
Shankaran S, Laptook AR, Ehrenkranz RA, et al.; National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84.
Veng-Pedersen P, Widness JA, Pereira LM, Schmidt RL, Lowe LS. A comparison of nonlinear pharmacokinetics of erythropoietin in sheep and humans. Biopharm Drug Dispos 1999;20:217–23.
Widness JA, Veng-Pedersen P, Peters C, Pereira LM, Schmidt RL, Lowe LS. Erythropoietin pharmacokinetics in premature infants: developmental, nonlinearity, and treatment effects. J Appl Physiol (1985) 1996;80:140–8.
Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics 2008;122:383–91.
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008;48:303–32.
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci 2013;102:2941–52.
Brendel K, Comets E, Laffont C, Mentré F. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn 2010;37:49–65.
Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 2008;90:154–66.
Zanelli S, Buck M, Fairchild K. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J Perinatol 2011;31:377–86.
Jelkmann W. The enigma of the metabolic fate of circulating erythropoietin (Epo) in view of the pharmacokinetics of the recombinant drugs rhEpo and NESP. Eur J Haematol 2002;69:265–74.
Nalbant D, Saleh M, Goldman FD, Widness JA, Veng-Pedersen P. Evidence of receptor-mediated elimination of erythropoietin by analysis of erythropoietin receptor mRNA expression in bone marrow and erythropoietin clearance during anemia. J Pharmacol Exp Ther 2010;333:528–32.
Chapel SH, Veng-Pedersen P, Schmidt RL, Widness JA. Receptor-based model accounts for phlebotomy-induced changes in erythropoietin pharmacokinetics. Exp Hematol 2001;29:425–31.
Roberts JK, Stockmann C, Ward RM, et al. Population pharmacokinetics of darbepoetin alfa in conjunction with hypothermia for the treatment of neonatal hypoxic-ischemic encephalopathy. Clin Pharmacokinet 2015;54:1237–44.
Warwood TL, Ohls RK, Lambert DK, et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol 2006;26:296–300.
Lee BF, Wang LW, Lin SH, et al. Tc-99m-HL91 imaging in the early detection of neuronal injury in a neonatal rat model of hypoxic ischemia. Crit Care Med 2012;40:1930–8.
Muramatsu K, Fukuda A, Togari H, Wada Y, Nishino H. Vulnerability to cerebral hypoxic-ischemic insult in neonatal but not in adult rats is in parallel with disruption of the blood-brain barrier. Stroke 1997;28:2281–8; discussion 2288–9.
Plateel M, Teissier E, Cecchelli R. Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem 1997;68:874–7.
Xenocostas A, Cheung WK, Farrell F, et al. The pharmacokinetics of erythropoietin in the cerebrospinal fluid after intravenous administration of recombinant human erythropoietin. Eur J Clin Pharmacol 2005;61:189–95.
Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate 2004;85:138–44.
Iwai M, Stetler RA, Xing J, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke 2010;41:1032–7.
Reitmeir R, Kilic E, Kilic U, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain 2011;134(Pt 1):84–99.
Larpthaveesarp A, Georgevits M, Ferriero DM, Gonzalez FF. Delayed erythropoietin therapy improves histological and behavioral outcomes after transient neonatal stroke. Neurobiol Dis 2016;93:57–63.
Baserga MC, Beachy JC, Roberts JK, et al. Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial. Pediatr Res 2015;78:315–22.
Acknowledgements
The authors thank Krisa Van Meurs, MD for her overall guidance and perspective in support of the study and Jessica Kan Vedder, BA, MPH for her organizational support of the study, for creating the study database, and for her work in monitoring enrollment and data collection procedures. The authors thank Zahra Afsharinejad, MS and Pat Janssen, MS for performing the MSD Epo assay and corresponding Epo data collection. The authors thank the following collaborators who enrolled patients or assisted with the design of the phase I and phase II studies: Roberta Ballard, MD, Sonia Bonifacio, MD, Taeun Chang, MD, Lawrence Dong, MD, David Durand, MD, Donna Ferriero, MD, Hannah Glass, MD, Fernando Gonzalez, MD, Amit Mathur, MD, Dennis Mayock, MD, MD, Sarah Mulkey, MD, Elizabeth Rogers, MD, Dongli Song, MD, Krisa Van Meurs, MD. The authors would also like to thank all the patients, families, and bedside nurses who participated in this study. Study data were collected and managed using REDCap electronic data capture tools hosted at University of California, San Francisco.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figure S1
(PDF 339 kb)
Rights and permissions
About this article
Cite this article
Frymoyer, A., Juul, S., Massaro, A. et al. High-dose erythropoietin population pharmacokinetics in neonates with hypoxic–ischemic encephalopathy receiving hypothermia. Pediatr Res 81, 865–872 (2017). https://doi.org/10.1038/pr.2017.15
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.15
This article is cited by
-
On target dosing: erythropoietin exposure in neonates with hypoxic-ischemic encephalopathy in the HEAL trial
Pediatric Research (2024)
-
New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy
European Journal of Pediatrics (2022)
-
Constant infusion case of one compartment pharmacokinetic model with simultaneous first-order and Michaelis–Menten elimination: analytical solution and drug exposure formula
Journal of Pharmacokinetics and Pharmacodynamics (2021)
-
Erythropoietin prevents necrotizing enterocolitis in very preterm infants: a randomized controlled trial
Journal of Translational Medicine (2020)
-
Theophylline dosing and pharmacokinetics for renal protection in neonates with hypoxic–ischemic encephalopathy undergoing therapeutic hypothermia
Pediatric Research (2020)