Abstract
Background:
Amino acid analysis is a valuable tool for cardiovascular risk assessment. Preterm infants display plasma amino acid changes in the newborn period. Whether these changes persist is unknown to date. The aim of this study was to assess whether former very preterm infants (VPI) show alterations in amino acid patterns indicative of an unfavorable cardiovascular risk profile at a preschool age.
Methods:
From 5—7 y-old children born at term or <32 wk gestation (VPI) were included in the study. Plasma amino acid concentrations were determined after an overnight fast.
Results:
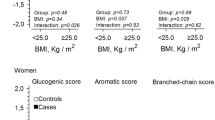
29 former term infants and 79 former VPI were included in the study. Former VPI showed changes in various plasma amino acids including glutamine, arginine, citrulline, tryptophan, glutamate, ornithine, and taurine. Branched-chain amino acids were lower, alanine/lysine ratios significantly higher in the preterm population.
Conclusion:
Former VPI show altered plasma amino acid profiles indicative of a dualistic cardiovascular risk profile (e.g., potentially beneficial elevations in citrulline, arginine, glutamine, and tryptophan, but also raised alanine/lysine ratios, low ornithine and taurine levels) at a preschool age. Whether this is associated with an adverse cardiovascular outcome has to be addressed by future studies. Long-term cardiometabolic follow-up of VPI might be warranted.
Similar content being viewed by others

Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med 2016;21:74–9.
Bayman E, Drake AJ, Piyasena C. Prematurity and programming of cardiovascular disease risk: a future challenge for public health? Archives of Disease in Childhood Fetal and Neonatal Edition 2014;99:F510–4.
Sipola-Leppänen M, Kajantie E. Should we assess cardiovascular risk in young adults born preterm? Curr Opin Lipidol 2015;26:282–7.
Posod A, Odri Komazec I, Kager K, Pupp Peglow U, Baumgartner D, Kiechl-Kohlendorfer U. PS-104early markers of an increased cardiovascular risk in former preterm tyrolean preschoolers—preliminary data. Archives of disease in childhood 2014;99:A148.
Odri Komazec I, Posod A, Schwienbacher M, et al. Aortic elastic properties in preschool children born preterm. Arterioscler Thromb Vasc Biol 2016;36:2268–74.
Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–31.
Rhee EP, Gerszten RE. Metabolomics and cardiovascular biomarker discovery. Clin Chem 2012;58:139–47.
Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012;55:321–30.
Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26.
Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53.
Magnusson M, Lewis GD, Ericson U, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J 2013;34:1982–9.
Mangge H, Zelzer S, Prüller F, et al. Branched-chain amino acids are associated with cardiometabolic risk profiles found already in lean, overweight, and obese young. J Nutr Biochem 2016;32:123–7.
Rigo J, Senterre J. Significance of plasma amino acid pattern in preterm infants. Biol Neonate 1987;52 Suppl 1:41–9.
Kromeyer-Hauschild K, Wabitsch M, Kunze D, Gellert F, Geiß HC, Hesse V. Percentiles of body mass index in children and adolescents evaluated from different regional German cohorts. Monatsschrift für Kinderheilkunde 2001:807–18.
Truthmann J, Mensink GB, Richter A. Relative validation of the KiGGS food frequency questionnaire among adolescents in Germany. Nutr J 2011;10:133.
Peplies J, Jiménez-Pavón D, Savva SC, et al.; IDEFICS consortium. Percentiles of fasting serum insulin, glucose, HbA1c and HOMA-IR in prepubertal normal weight European children from the IDEFICS cohort. Int J Obes (Lond) 2014;38 Suppl 2:S39–47.
Duran M. Amino acids. In: Blau N, Duran M, Gibson KM, eds. Laboratory Guide to the Methods in Biochemical Genetics. Berlin Heidelberg: Springer Verlag, 2008:53–89.
Corpeleijn WE, van den Akker CH, Roelants JA, van Goudoever JB. How proteins improve the development of preterm infants. Nestle Nutr Workshop Ser Pediatr Program 2011;68:33–45; discussion 45–8.
de Boo HA, Harding JE. Protein metabolism in preterm infants with particular reference to intrauterine growth restriction. Archives of Disease in Childhood Fetal and Neonatal Edition 2007;92:F315–9.
Barker DJ. Fetal and infant origins of adult disease. Monatsschrift Kinderheilkunde: Organ der Deutschen Gesellschaft fur Kinderheilkunde 2001;149:5.
Pencharz PB, Masson M, Desgranges F, Papageorgiou A. Total-body protein turnover in human premature neonates: effects of birth weight, intra-uterine nutritional status and diet. Clin Sci (Lond) 1981;61:207–15.
Piyasena C, Cartier J, Khulan B, et al. Dynamics of DNA methylation at IGF2 in preterm and term infants during the first year of life: an observational study. Lancet 2015;385 Suppl 1:S81.
Wu G, Morris SM Jr . Arginine metabolism: nitric oxide and beyond. Biochem J 1998;336 (Pt 1):1–17.
Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Clinical and pharmacogenetic impact of endothelial nitric oxide synthase polymorphisms on cardiovascular diseases. Nitric Oxide 2017;63:39–51.
Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006;113:1708–14.
Wu G, Collins JK, Perkins-Veazie P, et al. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr 2007;137:2680–5.
Popolo A, Adesso S, Pinto A, Autore G, Marzocco S. L-Arginine and its metabolites in kidney and cardiovascular disease. Amino Acids 2014;46:2271–86.
Li H, Meininger CJ, Bazer FW, Wu G. Intracellular sources of ornithine for polyamine synthesis in endothelial cells. Amino Acids 2016;48:2401–10.
Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 2016;22:1428–38.
Zheng Y, Hu FB, Ruiz-Canela M, et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvencion con DIeta MEDiterranea (PREDIMED) Trial. Journal of the American Heart Association 2016;5:e003755.
Zuo H, Ueland PM, Ulvik A, et al. Plasma biomarkers of inflammation, the Kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: the Hordaland health study. Am J Epidemiol 2016;183:249–58.
Polyzos KA, Ketelhuth DF. The role of the Kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie 2015;35:128–36.
Wannemacher RW Jr, Klainer AS, Dinterman RE, Beisel WR. The significance and mechanism of an increased serum phenylalanine-tyrosine ratio during infection. Am J Clin Nutr 1976;29:997–1006.
Murr C, Grammer TB, Meinitzer A, Kleber ME, März W, Fuchs D. Immune activation and inflammation in patients with cardiovascular disease are associated with higher phenylalanine to tyrosine ratios: the Ludwigshafen risk and cardiovascular health study. J Amino Acids 2014;2014:783730.
Katakawa M, Fukuda N, Tsunemi A, et al. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens Res 2016;39:848–56.
Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci 2010;17 Suppl 1:S6.
Takeshita H, Horiuchi M, Izumo K, et al. Long-term voluntary exercise, representing habitual exercise, lowers visceral fat and alters plasma amino acid levels in mice. Environ Health Prev Med 2012;17:275–84.
Hakuno D, Hamba Y, Toya T, Adachi T. Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS One 2015;10:e0117325.
Yanagisawa R, Kataoka M, Inami T, et al. Usefulness of circulating amino acid profile and Fischer ratio to predict severity of pulmonary hypertension. Am J Cardiol 2015;115:831–6.
McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes 2013;8:52–61.
Würtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation 2015;131:774–85.
Ho JE, Larson MG, Ghorbani A, et al. Metabolomic profiles of body mass index in the Framingham heart study reveal distinct cardiometabolic phenotypes. PLoS One 2016;11:e0148361.
Felig P, Marliss E, Cahill GF Jr . Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811–6.
Gamboa JL, Billings FTt, Bojanowski MT, et al. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiological Reports 2016;4:e12780.
Duicu OM, Lighezan R, Sturza A, et al. Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxid Med Cell Longev 2016;2016:8470394.
Chinnery PF. Mitochondrial disorders overview. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews(R). Seattle, WA: University of Washington, Seattle; 1993-2017.
Acknowledgements
The authors thank Katrin Kager, for her logistic and motivational support throughout the trial. The authors also thank Ulrike Eichinger-Öttl and Claudia Ertl, for performing all chromatographic analyses and kindly sharing their methodological expertise. Finally, the authors thank all children and their families for participating in and committing to our study.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Tables
(DOCX 56 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Posod, A., Müller, S., Komazec, I. et al. Former very preterm infants show alterations in plasma amino acid profiles at a preschool age. Pediatr Res 81, 787–794 (2017). https://doi.org/10.1038/pr.2017.24
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.24
This article is cited by
-
Metabolic profile changes in patients with rheumatoid arthritis detected using mass spectrometry
Scientific Reports (2025)
-
Effects of an exclusive human-milk diet in preterm neonates on early vascular aging risk factors (NEOVASC): study protocol for a multicentric, prospective, randomized, controlled, open, and parallel group clinical trial
Trials (2021)
-
Effects of nutrition therapy on growth, inflammation and metabolism in immature infants: a study protocol of a double-blind randomized controlled trial (ImNuT)
BMC Pediatrics (2021)